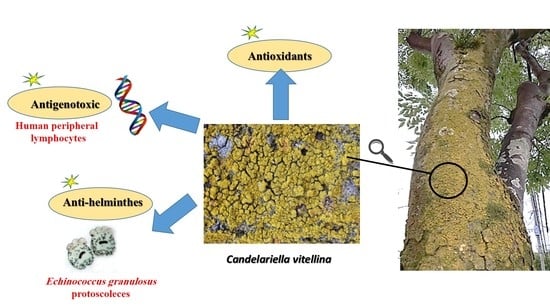
In Vitro Antigenotoxic, Antihelminthic and Antioxidant Potentials Based on the Extracted Metabolites from Lichen, Candelariella vitellina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Lichen Material
2.1.2. Genotoxic Drug
2.1.3. Antihelminthic Drug
2.2. Methods
2.2.1. Phytochemical Analyses
Preparation of the Extract
Estimation of Total Phenolic and Flavonoid Contents
Preliminary Qualitative Phenolic Analysis of the Extract
GC-MS Analysis
GC-MS Analysis of Silylated Metabolites
Qualitative Determination of Polyphenols Using HPLC Analysis
Antioxidant (Radical Scavenging Activity)
2.2.2. Cytotoxicity Studies on Normal Human Peripheral Blood Lymphocytes (HPBL)
HPBL Proliferation Assay (MTT Assay)
Cell Culture and Isolation
Acridine Orange/Ethidium Bromide (AO/EB) Dual Fluorescent Staining
Quantification of Apoptosis Using Annexin V/PI Labeling
Flow Cytometric Analysis of the Cell Cycle
2.2.3. Genotoxicity Studies on Normal Human Peripheral Blood Lymphocytes (HPBL)
Mitotic Index
prophases + non dividing cells)
Comet Assay
2.2.4. Antihelminthic Studies
Collection of Echinococcus granulosus Protoscoleces
Protoscoleces Maintenance and Treatments
Determination of Protoscoleces Viability
Scanning Electron Microscopy (SEM)
2.2.5. Data Analysis
3. Results
3.1. Phytochemical Analyses
3.1.1. Estimation of Total Phenolic (TPC) and Flavonoid (TFC) Contents
3.1.2. Preliminary Qualitative Analysis of the Extract
3.1.3. GC-MS Studies of Silylated Metabolites
3.1.4. Polyphenolics Analysis Using HPLC
3.1.5. Antioxidant Activities
3.2. Protective Effect of C. vitellina Extract
3.2.1. Cytotoxicity of C. vitellina on HPBL (MTT Assay)
3.2.2. Assessment of Viability by AO/EB Double Fluorescent Staining
3.2.3. Quantification of Apoptosis vs. Necrosis
3.2.4. Cell Cycle Analysis
3.3. Assessment of Genotoxicity
3.3.1. Mitotic Index
3.3.2. DNA Single-Strand Breaks
3.4. Antihelminthic Activities of C. vitellina Extract
3.4.1. Cytotoxicity of C. vitellina on E. granulosus Protoscoleces
3.4.2. Effect of C. vitellina Extract on E. granulosus Protoscoleces Ultrastructure
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Elkhateeb, W.A.; Daba, G.M. Lichens, an Alternative Drugs for Modern Diseases. Int. J. Res. Pharm. Biosci. 2019, 6, 5–9. [Google Scholar]
- Richardson, D. Medicinal and other economic aspects of lichens. CRC Handb. Lichenol. 1988, 3, 93–108. [Google Scholar]
- Kumar, K.C.; Müller, K. Lichen metabolites. 2. Antiproliferative and cytotoxic activity of gyrophoric, usnic, and diffractaic acid on human keratinocyte growth. J. Nat. Prod. 1999, 62, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Rankovič, B.; Rankovic, D.; Maric, D. Antioxidant and antimicrobial activity of some lichen species. Microbiology 2010, 79, 809–815. [Google Scholar] [CrossRef]
- Zambare, V.P.; Christopher, L.P. Biopharmaceutical potential of lichens. Pharm. Biol. 2012, 50, 778–798. [Google Scholar] [CrossRef]
- Tomović, J.; Kosanić, M.; Ristić, S.; Ranković, B.; Stanojković, T.; Manojlović, N. Chemical composition and bioactive properties of the lichen, Pleurosticta acetabulum. Trop. J. Pharm. Res. 2017, 16, 2977–2984. [Google Scholar] [CrossRef] [Green Version]
- Hawksworth, D.L. Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential. Lichenologist 2015, 47, 277–278. [Google Scholar] [CrossRef]
- Shibamoto, T.; Wei, C.L. Mutagenicity of lichen constituents. Environ. Mutagen. 1984, 6, 757–762. [Google Scholar] [CrossRef]
- Koparal, A.; Ayaz Tüylü, B.; Türk, H. In vitro cytotoxic activities of (+)-usnic acid and (−)-usnic acid on V79, A549, and human lymphocyte cells and their non-genotoxicity on human lymphocytes. Nat. Prod. Res. 2006, 20, 1300–1307. [Google Scholar] [CrossRef]
- Kristmundsdóttir, T.; Aradóttir, H.A.; Ingólfsdóttir, K.; Ögmundsdóttir, H.M. Solubilization of the lichen metabolite (+)-usnic acid for testing in tissue culture. J. Pharm. Pharmacol. 2002, 54, 1447–1452. [Google Scholar] [CrossRef]
- Koparal, A.T. Anti-angiogenic and antiproliferative properties of the lichen substances (-)-usnic acid and vulpinic acid. Z. Naturforschung C 2015, 70, 159–164. [Google Scholar] [CrossRef]
- Kotan, E.; Agar, G.; Alpsoy, L.; Aslan, A.; Erman, F.; Nardemir, G. Anti-genotoxic and anti-oxidative effects of Cladonia rangiformis extracts against aflatoxin B1 in vitro. Fresenius Environ. Bull. 2013, 22, 1139–1143. [Google Scholar]
- Ceker, S.; Orhan, F.; Sezen, S.; Gulluce, M.; Ozkan, H.; Aslan, A.; Agar, G. Anti-mutagenic and anti-oxidant potencies of Cetraria aculeata (Schreb.) Fr., Cladonia chlorophaea (Flörke ex sommerf.) spreng. and Cetrelia olivetorum (Nyl.) WL Culb. & CF Culb. Iran. J. Pharm. Res. IJPR 2018, 17, 326–335. [Google Scholar] [PubMed]
- Alpsoy, L.; Aslan, A.; Kotan, E.; Agar, G.; Anar, M. Protective role of two lichens in human lymphocytes in vitro. Fresenius Environ. Bull. 2011, 20, 1661–1666. [Google Scholar]
- Leandro, L.F.; Munari, C.C.; Sato, V.L.F.L.; Alves, J.M.; de Oliveira, P.F.; Mastrocola, D.F.P.; Martins, S.D.P.L.; da Silva Moraes, T.; de Oliveira, A.I.; Tozatti, M.G. Assessment of the genotoxicity and antigenotoxicity of (+)-usnic acid in V79 cells and Swiss mice by the micronucleus and comet assays. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 753, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Prokop’ev, I.; Filippov, E.; Filippova, G.; Zhanataev, A. Pro/antigenotoxic activity of usnic acid enantiomers in vitro. Bull. Exp. Biol. Med. 2018, 164, 312–315. [Google Scholar] [CrossRef]
- Geyikoglu, F.; Turkez, H.; Aslan, A. The protective roles of some lichen species on colloidal bismuth subcitrate genotoxicity. Toxicol. Ind. Health 2007, 23, 487–492. [Google Scholar] [CrossRef]
- Sachindra, N.; Airanthi, M.; Hosokawa, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J. Food Sci. Technol. 2010, 47, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Wei, X.; Yamamoto, Y.; Liu, Y.; Wang, L.; Jung, J.S.; Koh, Y.J.; Hur, J.-S. Antioxidant activities of edible lichen Ramalina conduplicans and its free radical-scavenging constituents. Mycoscience 2010, 51, 391–395. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Vukojević, J. Antioxidant properties of some lichen species. J. Food Sci. Technol. 2011, 48, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Plaza, C.M.; de Torres, L.E.D.; Lücking, R.K.; Vizcaya, M.; Medina, G.E. Antioxidant activity, total phenols and flavonoids of lichens from Venezuelan Andes. J. Pharm. Pharmacogn. Res. 2014, 2, 138–147. [Google Scholar]
- Paudel, B.; Bhattarai, H.D.; Koh, H.Y.; Lee, S.G.; Han, S.J.; Lee, H.K.; Oh, H.; Shin, H.W.; Yim, J.H. Ramalin, a novel nontoxic antioxidant compound from the Antarctic lichen Ramalina terebrata. Phytomedicine 2011, 18, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- El-Garawani, I.M.; Elkhateeb, W.A.; Zaghlol, G.M.; Almeer, R.S.; Ahmed, E.F.; Rateb, M.E.; Moneim, A.E.A. Candelariella vitellina extract triggers in vitro and in vivo cell death through induction of apoptosis: A novel anticancer agent. Food Chem. Toxicol. 2019, 127, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkhateeb, W.A.; Daba, G.M.; Negm El-Dein, A.; Sheir, D.; Fayad, W.; Shaheen, M.N.; Elmahdy, M.E.; Wen, T.C. Insights into the in vitro hypocholesterolemic, antioxidant, anti-rotavirus, and anti-colon cancer, activities of the methanolic extracts of a Japanese lichen, Candelariella vitellina, and a Japanese mushroom, Ganoderma applanatum. Egypt. Pharm. J. 2020, 19, 67–73. [Google Scholar]
- Aziz, K.; Nowsheen, S.; Pantelias, G.; Iliakis, G.; Gorgoulis, V.; Georgakilas, A. Targeting DNA damage and repair: Embracing the pharmacological era for successful cancer therapy. Pharmacol. Ther. 2012, 133, 334–350. [Google Scholar] [CrossRef]
- Colombo, P.; Gunnarsson, K.; Iatropoulos, M.; Brughera, M. Toxicological testing of cytotoxic drugs. Int. J. Oncol. 2001, 19, 1021–1028. [Google Scholar]
- Weijl, N.; Cleton, F.; Osanto, S. Free radicals and antioxidants in chemotherapyinduced toxicity. Cancer Treat. Rev. 1997, 23, 209–240. [Google Scholar] [CrossRef]
- Iwao, K.; Inatani, M.; Seto, T.; Takihara, Y.; Ogata-Iwao, M.; Okinami, S.; Tanihara, H. Long-term outcomes and prognostic factors for trabeculectomy with mitomycin C in eyes with uveitic glaucoma: A retrospective cohort study. J. Glaucoma 2014, 23, 88–94. [Google Scholar] [CrossRef]
- Dorr, R.T.; Bowden, G.T.; Alberts, D.S.; Liddil, J.D. Interactions of mitomycin C with mammalian DNA detected by alkaline elution. Cancer Res. 1985, 45, 3510–3516. [Google Scholar]
- Dusre, L.; Covey, J.M.; Collins, C.; Sinha, B.K. DNA damage, cytotoxicity and free radical formation by mitomycin C in human cells. Chem.-Biol. Interact. 1989, 71, 63–78. [Google Scholar] [CrossRef]
- Turkez, H.; Aydin, E.; Aslan, A. Xanthoria elegans (Link)(lichen) extract counteracts DNA damage and oxidative stress of mitomycin C in human lymphocytes. Cytotechnology 2012, 64, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Roh, D.S.; Cook, A.L.; Rhee, S.S.; Joshi, A.; Kowalski, R.; Dhaliwal, D.K.; Funderburgh, J.L. DNA cross-linking, double-strand breaks, and apoptosis in corneal endothelial cells after a single exposure to mitomycin C. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4837–4843. [Google Scholar] [CrossRef] [PubMed]
- Unal, F.; Taner, G.; Yuzbasioglu, D.; Yilmaz, S. Antigenotoxic effect of lipoic acid against mitomycin-C in human lymphocyte cultures. Cytotechnology 2013, 65, 553–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, J.H.; Wang, H.; Liu, R.-M.; Liu, J.; Hagen, T.M. (R)-α-Lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: Evidence for increased cysteine requirement for GSH synthesis. Arch. Biochem. Biophys. 2004, 423, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef]
- Rajabi, M.A. Fatal reactions and methaemoglobinaemia after silver nitrate irrigation of hydatid cyst. Surg. Pract. 2009, 13, 2–7. [Google Scholar] [CrossRef]
- Sahin, M.; Eryilmaz, R.; Bulbuloglu, E. The effect of scolicidal agents on liver and biliary tree (experimental study). J. Investig. Surg. 2004, 17, 323–326. [Google Scholar] [CrossRef]
- Hemphill, A.; Walker, M. Drugs against echinococcosis. Drug Des. Rev.-Online 2004, 1, 325–332. [Google Scholar] [CrossRef]
- Khalkhali, H.; Foroutan, M.; Khademvatan, S.; Majidiani, H.; Aryamand, S.; Khezri, P.; Aminpour, A. Prevalence of cystic echinococcosis in Iran: A systematic review and meta-analysis. J. Helminthol. 2018, 92, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef]
- Galeh, T.M.; Spotin, A.; Mahami-Oskouei, M.; Carmena, D.; Rahimi, M.T.; Barac, A.; Ghoyounchi, R.; Berahmat, R.; Ahmadpour, E. The seroprevalence rate and population genetic structure of human cystic echinococcosis in the Middle East: A systematic review and meta-analysis. Int. J. Surg. 2018, 51, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Haleem, S.; Niaz, S.; Qureshi, N.A.; Ullah, R.; Mahmood, H.M.; Shahat, A.A. Phytochemical analysis, Antioxidant and Antiprotoscolices potential of ethanol extracts of selected plants species against Echinococcus granulosus: In-vitro study. Open Chem. 2019, 17, 874–883. [Google Scholar] [CrossRef]
- El-Garawani, I.M.; El-Nabi, S.E.H.; Mohamed, A.H.; El-Esawy, H.M. Molecular amelioration of Acacia arabica Gum on some male reproductive aspects in Schistosoma mansoni infected mice. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 505–512. [Google Scholar]
- Saeed, M.; Amen, A.; Fahmi, A.; El Garawani, I.; Sayed, S. The possible protective effect of Coriandrum sativum seeds methanolic extract on hepato-renal toxicity induced by sodium arsenite in albino rats. J. Appl. Pharm. Sci. 2014, 4, 44–51. [Google Scholar]
- Sakr, S.; Sobhy, E.; Yosry, A.; Islam, M. Cytoprotective effects of aqueous ginger (Zingiber officinale) extract against carbimazole-induced toxicity in albino rats. Ejpmr 2016, 3, 489–497. [Google Scholar]
- El-Garawani, I.; Hassab El Nabi, S.; El-Ghandour, E. The protective effect of (Foeniculum vulgare) oil on etoposide-induced genotoxicity on male albino rats. Eur. J. Pharm. Med. Res. 2017, 4, 180–194. [Google Scholar]
- El-Garawani, I.M. Ameliorative effect of Cymbopogon citratus extract on cisplatin-induced genotoxicity in human leukocytes. Biol. Sci. Appl. Res. 2015, 1, 304–310. [Google Scholar]
- Tohamy, A.A.; El-Garawani, I.M.; Ibrahim, S.R.; Moneim, A.E.A. The apoptotic properties of Salvia aegyptiaca and Trigonella foenum-graecum extracts on Ehrlich ascites carcinoma cells: The effectiveness of combined treatment. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1872–1883. [Google Scholar]
- Ahmed, A.; Ali, M.; El-Kholie, E.; El-Garawani, I.; Sherif, N. Anticancer activity of Morus nigra on human breast cancer cell line (MCF-7): The role of fresh and dry fruit extracts. J. Biosci. Appl. Res. 2016, 2, 352–361. [Google Scholar]
- El-Nabi, S.H.; Dawoud, G.; El-Garawani, I.; El-Shafey, S. HPLC analysis of phenolic acids, antioxidant activity and in vitro effectiveness of green and roasted Caffea arabica bean extracts: A comparative study. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2018, 18, 1281–1288. [Google Scholar] [CrossRef]
- Elkhateeb, W.A.; Zaghlol, G.M.; El-Garawani, I.M.; Ahmed, E.F.; Rateb, M.E.; Moneim, A.E.A. Ganoderma applanatum secondary metabolites induced apoptosis through different pathways: In vivo and in vitro anticancer studies. Biomed. Pharmacother. 2018, 101, 264–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Garawani, I.; El Nabi, S.H.; Nafie, E.; Almeldin, S. Foeniculum vulgare and Pelargonium graveolens Essential Oil Mixture Triggers the Cell Cycle Arrest and Apoptosis in MCF-7 Cells. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2019, 19, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- El-Garawani, I.M.; El-Nabi, S.H.; El-Shafey, S.; Elfiky, M.; Nafie, E. Coffea arabica Bean Extracts and Vitamin C: A Novel Combination Unleashes MCF-7 Cell Death. Curr. Pharm. Biotechnol. 2020, 21, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Dubino, A. Lichens as a source of chemical compounds with anti-inflammatory activity. Herba Pol. 2018, 64, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Žilić, S.; Serpen, A.; Akıllıoğlu, G.L.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar]
- Kokate, C.; Purohit, A.; Gokhale, S. Carbohydrate and derived Products, drugs containing glycosides, drugs containing tannins, lipids and protein alkaloids. Text Book Pharmacogn. 2001, 7, 133–166. [Google Scholar]
- Harborne, A. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Haddock, E.A.; Gupta, R.K.; Al-Shafi, S.M.; Haslam, E.; Magnolato, D. The metabolism of gallic acid and hexahydroxydiphenic acid in plants. Part 1. Introduction. Naturally occurring galloyl esters. J. Chem. Soc. Perkin Trans. 1982, 1, 2515–2524. [Google Scholar] [CrossRef]
- Bate-Smith, E. Detection and determination of ellagitannins. Phytochemistry 1972, 11, 1153–1156. [Google Scholar] [CrossRef]
- Wilson, T.C.; Hagerman, A.E. Quantitative determination of ellagic acid. J. Agric. Food Chem. 1990, 38, 1678–1683. [Google Scholar] [CrossRef]
- Farag, M.A.; Mohsen, E.; Abd El Nasser, G. Sensory metabolites profiling in Myristica fragrans (Nutmeg) organs and in response to roasting as analyzed via chemometric tools. LWT 2018, 97, 684–692. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Do Thi, N. Effects of extraction and processing methods on antioxidant compound contents and radical scavenging activities of laver (Porphyra tenera). Prev. Nutr. Food Sci. 2014, 19, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Oktay, M.; Gülçin, İ.; Küfrevioğlu, Ö.İ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Liu, K.; Liu, P.-C.; Liu, R.; Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 2015, 21, 15–20. [Google Scholar]
- Evans, H. Cytological methods for detecting chemical mutagens. In Chemical Mutagens; Springer: Berlin, Germany, 1976; pp. 1–29. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudvand, H.; Jahanbakhsh, S.; Nadri, S.; Mahmoudvand, H. Inhibitory activity of Fennel methanolic extract against hydatid cyst protoscoleces. J. Chem. Pharm. Sci. 2016, 9, 2500–2503. [Google Scholar]
- Barabadi, H.; Honary, S.; Mohammadi, M.A.; Ahmadpour, E.; Rahimi, M.T.; Alizadeh, A.; Naghibi, F.; Saravanan, M. Green chemical synthesis of gold nanoparticles by using Penicillium aculeatum and their scolicidal activity against hydatid cyst protoscolices of Echinococcus granulosus. Environ. Sci. Pollut. Res. 2017, 24, 5800–5810. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Shakib, S.S.; Sameni, A.K.; Taghiabadi, E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran. J. Pharm. Res. IJPR 2013, 12, 93–99. [Google Scholar] [CrossRef]
- Sadjjadi, S.M.; Zoharizadeh, M.R.; Panjeshahin, M.R. In vitro screening of different Allium sativum extracts on hydatid cysts protoscoleces. J. Investig. Surg. 2008, 21, 318–322. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, Y.; Wang, Z.; Li, F.; Xing, G.; Peng, X.; Zhang, S.; Lv, H. Arsenic trioxide negatively affects Echinococcus granulosus. Antimicrob. Agents Chemother. 2015, 59, 6946–6951. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.P. UV-Vis spectrophotometry plus HPLC to measure the level of catechin/poly–phenolics and to understand its oxidized conditions in commercially available green and black teas. Indian J. Chem. Sect. B 2014, 53, 1255–1262. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giada, M. Food phenolic compounds: Main classes, sources and their antioxidant power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Intech: London, UK, 2013; pp. 87–112. [Google Scholar]
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant activity of caffeic acid against iron-induced free radical generation—A chemical approach. PLoS ONE 2015, 10, e0129963. [Google Scholar]
- De Beer, D.; Joubert, E.; Gelderblom, W.; Manley, M. Phenolic compounds: A review of their possible role as in vivo antioxidants of wine. S. Afr. J. Enol. Vitic. 2002, 23, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Macheix, J.-J. Fruit Phenolics: 0; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Kerry, N.L.; Abbey, M. Red wine and fractionated phenolic compounds prepared from red wine inhibit low density lipoprotein oxidation in vitro. Atherosclerosis 1997, 135, 93–102. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity–structure relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Uskova, M.; Kravchenko, L. Antioxidant properties of lactic acid bacteria—Probiotic and yogurt strains. Vopr. Pitan. 2009, 78, 18–23. [Google Scholar]
- Seki, T.; Morimura, S.; Tabata, S.; Tang, Y.; Shigematsu, T.; Kida, K. Antioxidant activity of vinegar produced from distilled residues of the Japanese liquor shochu. J. Agric. Food Chem. 2008, 56, 3785–3790. [Google Scholar] [CrossRef]
- Henry, G.E.; Momin, R.A.; Nair, M.G.; Dewitt, D.L. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 2002, 50, 2231–2234. [Google Scholar] [CrossRef]
- Lopez, S.; Bermudez, B.; Pacheco, Y.M.; Ortega, A.; Varela, L.M.; Abia, R.; Muriana, F.J. Oleic Acid: The Main Component of Olive Oil on Postprandial Metabolic Processes. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1385–1393. [Google Scholar]
- Wang, Z.J.; Liang, C.L.; Li, G.M.; Yu, C.Y.; Yin, M. Stearic acid protects primary cultured cortical neurons against oxidative stress 4. Acta Pharmacol. Sin. 2007, 28, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Van den Ende, W.; Peshev, D. Sugars as antioxidants in plants. In Crop Improvement under Adverse Conditions; Springer: Berlin, Germany, 2013; pp. 285–307. [Google Scholar]
- Tapas, A.R.; Sakarkar, D.; Kakde, R. Flavonoids as nutraceuticals: A review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Aksoy, L.; Kolay, E.; Ağılönü, Y.; Aslan, Z.; Kargıoğlu, M. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J. Biol. Sci. 2013, 20, 235–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanduja, K.L.; Avti, P.K.; Kumar, S.; Mittal, N.; Sohi, K.K.; Pathak, C.M. Anti-apoptotic activity of caffeic acid, ellagic acid and ferulic acid in normal human peripheral blood mononuclear cells: A Bcl-2 independent mechanism. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ferk, F.; Chakraborty, A.; Jäger, W.; Kundi, M.; Bichler, J.; Mišík, M.; Wagner, K.-H.; Grasl-Kraupp, B.; Sagmeister, S.; Haidinger, G. Potent protection of gallic acid against DNA oxidation: Results of human and animal experiments. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011, 715, 61–71. [Google Scholar] [CrossRef]
- Schewe, T.; Steffen, Y.; Sies, H. How do dietary flavanols improve vascular function? A position paper. Arch. Biochem. Biophys. 2008, 476, 102–106. [Google Scholar] [CrossRef]
- Thresiamma, K.; George, J.; Kuttan, R. Protective effect of curcumin, ellagic acid and bixin on radiation induced genotoxicity. J. Exp. Clin. Cancer Res. CR 1998, 17, 431–434. [Google Scholar]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef]
- Abraham, S.K.; Sarma, L.; Kesavan, P. Protective effects of chlorogenic acid, curcumin and β-carotene against γ-radiation-induced in vivo chromosomal damage. Mutat. Res. Lett. 1993, 303, 109–112. [Google Scholar] [CrossRef]
- Devipriya, N.; Sudheer, A.R.; Menon, V.P. Caffeic acid protects human peripheral blood lymphocytes against gamma radiation-induced cellular damage. J. Biochem. Mol. Toxicol. 2008, 22, 175–186. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J.; Azadbakht, M.; Tanha, M.; Mahmodzadeh, A.; Mohammadifar, S. Protective effect of hawthorn extract against genotoxicity induced by methyl methanesulfonate in human lymphocytes. Toxicol. Ind. Health 2011, 27, 363–369. [Google Scholar] [CrossRef]
- Sivas, H. Antigenotoxic effect of some lichen metabolites. In Lichen Secondary Metabolites; Springer: Berlin, Germany, 2019; pp. 175–197. [Google Scholar]
- Loos, J.A.; Cumino, A.C. In vitro anti-echinococcal and metabolic effects of metformin involve activation of AMP-activated protein kinase in larval stages of Echinococcus granulosus. PLoS ONE 2015, 10, e0126009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.C.; Gangwar, M.; Yashpal, M.; Nath, G. Anticestodal activity of endophytic Pestalotiopsis sp. on protoscoleces of hydatid cyst Echinococcus granulosus. BioMed Res. Int. 2013, 2013, 308515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef] [Green Version]
- Moazeni, M.; Larki, S.; Oryan, A.; Saharkhiz, M.J. Preventive and therapeutic effects of Zataria multiflora methanolic extract on hydatid cyst: An in vivo study. Vet. Parasitol. 2014, 205, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Larki, S.; Jalali, M.H.R.; Goodarzi, S. Scolicidal Effects of Gallic Acid, One of the Major Compounds of Plants, on Protoscolices of Hydatid Cyst. Zahedan J. Res. Med. Sci. 2017, 19, e9791. [Google Scholar] [CrossRef] [Green Version]








| Test | Observed Color | Conclusion |
|---|---|---|
| Ferric chloride FeCl3 (1%) | Intense green (+ve) | A major presence of phenolics |
| Shinoda’s (Mg/conc. HCl) | Light red (+ve) | Light presence of flavonoids and/or their glycosides |
| Potassium Iodate (KIO3) | −ve | Absent of gallotannins |
| Sodium nitrite (NaNO2) | −ve | Absent of ellagitannins |
| Category | Compound Name | Molecular Weight | Molecular Formula | Rt (min) |
|---|---|---|---|---|
| Acids | 2-hydroxy Propanoic acid (D-Lactic Acid) | 90 | C3H6O3 | 4.14 |
| Acetic acid | 152 | C8H8O3 | 4.48 | |
| Dodecanoic acid (Lauric acid) | 200 | C12H24O2 | 15.04 | |
| 3,4 dihydroxy Benzoic acid (Protocatechuic acid) | 154 | C7H6O4 | 17.51 | |
| Tetradecanoic acid (Myristic acid) | 228 | C₁₄H₂₈O₂ | 18.08 | |
| Hexadecanoic acid (Palmitic acid) | 256 | C16H32O2 | 20.85 | |
| Octadecanoic acid (Stearic Acid) | 284 | C18H36O2 | 23.43 | |
| Alcohols | Glycerol | 92 | C3H8O3 | 7.99 |
| Butane-2,3-diol | 90 | C4H10O2 | 12.58 | |
| Ethane-1,2-diol (Ethylene glycol) | 62 | C2H6O2 | 12.94 | |
| Sugars | Arabinofuranose | 150 | C5H10O5 | 13.02 |
| Xylonic acid | 166 | C5H10O6 | 14.37 | |
| D-(+)-Ribono-1,4-lactone | 148 | C5H8O5 | 14.53 | |
| α-Xylopyranose | 150 | C5H10O5 | 14.84–15.13 | |
| Xylitol | 152 | C₅H₁₂O₅ | 15.35 | |
| Methyl-α-D-galactopyranoside | 194 | C7H14O6 | 17.75 | |
| D-glucose | 180 | C6H12O6 | 18.16 | |
| α-L-Arabinopyranose | 150 | C5H10O5 | 18.35 | |
| Methyl-α-D-glucopyranoside | 194 | C7H14O6 | 18.41 | |
| Erythritol | 122 | C4H10O4 | 18.59 | |
| Ethyl-α-D-galactofuranoside | 208 | C8H16O6 | 19.24 | |
| Total identified % is 71.23 and SI ≥ 700 | ||||
| No. | Identified Metabolites | Rt (min) | Area | Conc. (µg/g) |
|---|---|---|---|---|
| 1 | Gallic acid | 3.309 | 1094.90 | 3160.53 |
| 2 | Chlorogenic acid | 4.105 | 1513.29 | 4395.52 |
| 3 | Caffeic acid | 5.740 | 905.23 | 1209.61 |
| 4 | Syringic acid | 6.505 | 580.31 | 767.17 |
| 5 | Pyro catechol | 6.814 | 28.25 | 100.60 |
| 6 | Rutin | 7.324 | 133.15 | 684.75 |
| 7 | Ellagic acid | 8.103 | 32.20 | 70.18 |
| 8 | Taxifolin | 12.455 | 79.71 | 261.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Garawani, I.; Emam, M.; Elkhateeb, W.; El-Seedi, H.; Khalifa, S.; Oshiba, S.; Abou-Ghanima, S.; Daba, G. In Vitro Antigenotoxic, Antihelminthic and Antioxidant Potentials Based on the Extracted Metabolites from Lichen, Candelariella vitellina. Pharmaceutics 2020, 12, 477. https://doi.org/10.3390/pharmaceutics12050477
El-Garawani I, Emam M, Elkhateeb W, El-Seedi H, Khalifa S, Oshiba S, Abou-Ghanima S, Daba G. In Vitro Antigenotoxic, Antihelminthic and Antioxidant Potentials Based on the Extracted Metabolites from Lichen, Candelariella vitellina. Pharmaceutics. 2020; 12(5):477. https://doi.org/10.3390/pharmaceutics12050477
Chicago/Turabian StyleEl-Garawani, Islam, Mahmoud Emam, Waill Elkhateeb, Hesham El-Seedi, Shaden Khalifa, Salwa Oshiba, Shaimaa Abou-Ghanima, and Ghoson Daba. 2020. "In Vitro Antigenotoxic, Antihelminthic and Antioxidant Potentials Based on the Extracted Metabolites from Lichen, Candelariella vitellina" Pharmaceutics 12, no. 5: 477. https://doi.org/10.3390/pharmaceutics12050477