Abstract
Background:
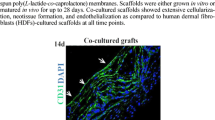
Introduction of pro-angiogenic cells into tissue-engineered (TE) constructs (prevascularisation) is a promising approach to overcome delayed neovascularisation of such constructs post-implantation. Accordingly, in this study, we examined the contribution of human dermal microvascular endothelial cells (HDMECs) and human dermal fibroblasts (HDFs) alone and in combination on the formation of new blood vessels in ex-ovo chick chorioallantoic membrane (CAM) assay.
Methods:
Poly-3-hydroxybutyrate-co-3-hydroxyvalerate (PHBV) and polycaprolactone (PCL) were first examined in terms of their physical, mechanical, and biological performances. The effect of gelatin coating and co-culture conditions on enhancing endothelial cell viability and growth was then investigated. Finally, the angiogenic potential of HDMECs and HDFs were assessed macroscopically and histologically after seeding on simple electrospun PHBV scaffolds either in isolation or in indirect co-culture using an ex-ovo CAM assay.
Results:
The results demonstrated that PHBV was slightly more favourable than PCL for HDMECs in terms of cell metabolic activity. The gelatin coating of PHBV scaffolds and co-culture of HDMECs with HDFs both showed a positive impact on HDMECs viability and growth. Both cell types induced angiogenesis over 7 days in the CAM assay either in isolation or in co-culture. The introduction of HDMECs to the scaffolds resulted in the production of more blood vessels in the area of implantation than the introduction of HDFs, but the co-culture of HDMECs and HDFs gave the most significant angiogenic activity.
Conclusion:
Our findings showed that the in vitro prevascularisation of TE constructs with HDMECs and HDFs alone or in co-culture promotes angiogenesis in implantable TE constructs.






Similar content being viewed by others
References
Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6.
Langer R. Tissue engineering. Mol Ther. 2000;1:12–5.
Rivron NC, Liu J, Rouwkema J, De Boer J, Van Blitterswijk CA. Engineering vascularised tissues in vitro. Eur Cells Mater. 2008;15:27–40.
Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434–41.
Rouwkema J, Koopman B, Blitterswijk C, Dhert W, Malda J. Supply of nutrients to cells in engineered tissues. Biotechnol Genet Eng Rev. 2009;26:163–78.
Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300–11.
Duttenhoefer F, Lara de Freitas R, Meury T, Loibl M, Benneker LM, Richards RG, et al. 3D scaffolds co-seeded with human endothelial progenitor and mesenchymal stem cells: evidence of prevascularisation within 7 days. Eur Cells Mater. 2013;26:49–65.
Yow KH, Ingram J, Korossis SA, Ingham E, Homer-Vanniasinkam S. Tissue engineering of vascular conduits. Br J Surg. 2006;93:652–61.
Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vascularization–the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009;15:159–69.
Quillaguamán J, Guzmán H, Van-Thuoc D, Hatti-Kaul R. Synthesis and production of polyhydroxyalkanoates by halophiles: current potential and future prospects. Appl Microbiol Biotechnol. 2010;85:1687–96.
Aldemir Dikici B, Sherborne C, Reilly GC, Claeyssens F. Emulsion templated scaffolds manufactured from photocurable polycaprolactone. Polymer (Guildf). 2019;175:243–54.
Aldemir Dikici B, Reilly GC, Claeyssens F. Boosting the osteogenic and angiogenic performance of multiscale porous polycaprolactone scaffolds by in vitro generated extracellular matrix decoration. ACS Appl Mater Interfaces. 2020;12:12510–24.
Bezwada RS, Jamiolkowski DD, Lee IY, Agarwal V, Persivale J, Trenka-Benthin S, et al. Monocryl® suture, a new ultra-pliable absorbable monofilament suture. Biomaterials. 1995;16:1141–8.
Darney PD, Monroe SE, Klaisle CM, Alvarado A. Clinical evaluation of the Capronor contraceptive implant: preliminary report. Am J Obstet Gynecol. 1989;160:1292–5.
Bye FJ, Bissoli J, Black L, Bullock AJ, Puwanun S, Moharamzadeh K, et al. Development of bilayer and trilayer nanofibrous/microfibrous scaffolds for regenerative medicine. Biomater Sci. 2013;1:942–51.
Bye FJ, Wang L, Bullock AJ, Blackwood KA, Ryan AJ, Macneil S. Postproduction processing of electrospun fibres for tissue engineering. J Vis Exp. 2012;66:4172.
Aldemir Dikici B, Dikici S, Reilly GC, MacNeil S, Claeyssens F. A novel bilayer polycaprolactone membrane for guided bone regeneration: combining electrospinning and emulsion templating. Materials (Basel). 2019;12:E2643.
Dikici S, Mangır N, Claeyssens F, Yar M, MacNeil S. Exploration of 2-deoxy-d-ribose and 17β-Estradiol as alternatives to exogenous VEGF to promote angiogenesis in tissue-engineered constructs. Regen Med. 2019;14:179–97.
Dikici S, Claeyssens F, MacNeil S. Decellularised baby spinach leaves and their potential use in tissue engineering applications: studying and promoting neovascularisation. J Biomater Appl. 2019;34:546–59.
Mangir N, Dikici S, Claeyssens F, MacNeil S. Using ex Ovo Chick Chorioallantoic Membrane (CAM) assay to evaluate the biocompatibility and angiogenic response to biomaterials. ACS Biomater Sci Eng. 2019;5:3190–200.
Goel MK, Khanna P, Kishore J. Understanding survival analysis: Kaplan–Meier estimate. Int J Ayurveda Res. 2010;1:274–8.
Mangır N, Hillary CJ, Chapple CR, MacNeil S. Oestradiol-releasing biodegradable mesh stimulates collagen production and angiogenesis: an approach to improving biomaterial integration in pelvic floor repair. Eur Urol Focus. 2019;5:280–9.
Minajeva A, Kase M, Saretok M, Adamson-Raieste A, Kase S, Niinepuu K, et al. Impact of blood vessel quantity and vascular expression of CD133 and ICAM-1 on survival of glioblastoma patients. Neurosci J. 2017;2017:5629563.
Wang L, Du J, Cao D, Wang Y. Recent advances and the application of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) as tissue engineering materials. J M S Pure Appl Chem. 2013;50:885–93.
Dikici S, Aldemir Dikici B, Bhaloo SI, Balcells M, Edelman ER, MacNeil S, et al. Assessment of the angiogenic potential of 2-deoxy-d-ribose using a novel in vitro 3D dynamic model in comparison with established in vitro assays. Front Bioeng Biotechnol. 2019;7:451.
Sangsanoh P, Waleetomcheepsawat S, Suwantong O, Wutticharoenmongkol P, Weeranantanapan O, Chuenjitbuntaworn B, et al. In vitro biocompatability of Schwann cells on surfaces of biocompatible polymeric electrospun fibrous and solution-cast film scaffolds. Biomacromolecules. 2007;8:1587–94.
Alagoz AS, Rodriguez-Cabello JC, Hasirci V. PHBV wet-spun scaffold coated with ELR-REDV improves vascularization for bone tissue engineering. Biomed Mater. 2018;13:055010.
Lei C, Zhu H, Li J, Li J, Feng X, Chen J. Preparation and characterization of polyhydroxybutyrate-co-hydroxyvalerate/silk fibroin nanofibrous scaffolds for skin tissue engineering. Polym Eng Sci. 2015;55:907–16.
Jalali S, Tafazzoli-Shadpour M, Haghighipour N, Omidvar R, Safshekan F. Regulation of endothelial cell adherence and elastic modulus by substrate stiffness. Cell Commun Adhes. 2015;22:79–89.
Ataollahi F, Pramanik S, Moradi A, Dalilottojari A, Pingguan-Murphy B, Wan Abas WA, et al. Endothelial cell responses in terms of adhesion, proliferation, and morphology to stiffness of polydimethylsiloxane elastomer substrates. J Biomed Mater Res A. 2015;103:2203–13.
Rüder C, Sauter T, Kratz K, Haase T, Peter J, Jung F, et al. Influence of fibre diameter and orientation of electrospun copolyetheresterurethanes on smooth muscle and endothelial cell behaviour. Clin Hemorheol Microcirc. 2013;55:513–22.
Ko YG, Park JH, Lee JB, Oh HH, Park WH, Cho D, et al. Growth behavior of endothelial cells according to electrospun poly(d,l-lactic-co-glycolic acid) fiber diameter as a tissue engineering scaffold. Tissue Eng Regen Med. 2016;13:343–51.
Stoker MG, Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967;215:171–2.
Frame KK, Hu WS. A model for density-dependent growth of anchorage-dependent mammalian cells. Biotechnol Bioeng. 1988;32:1061–6.
Tremel A, Cai A, Tirtaatmadja N, Hughes BD, Stevens GW, Landman KA, et al. Cell migration and proliferation during monolayer formation and wound healing. Chem Eng Sci. 2009;64:247–53.
De Silva Thompson D, Peticone C, Burova I, Shipley RJ, Knowles JC, Kim HW, et al. Assessing behaviour of osteoblastic cells in dynamic culture conditions using titanium-doped phosphate glass microcarriers. J Tissue Eng. 2019;10:2041731419825772.
Lerman MJ, Lembong J, Muramoto S, Gillen G, Fisher JP. The evolution of polystyrene as a cell culture material. Tissue Eng Part B Rev. 2018;24:5359–72.
Lannutti J, Reneker D, Ma T, Tomasko D, Farson D. Electrospinning for tissue engineering scaffolds. Mater Sci Eng C Mater Biol Appl. 2007;27:504–9.
Blackwood KA, McKean R, Canton I, Freeman CO, Franklin KL, Cole D, et al. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials. 2008;29:3091–104.
Szentivanyi A, Chakradeo T, Zernetsch H, Glasmacher B. Electrospun cellular microenvironments: understanding controlled release and scaffold structure. Adv Drug Deliv Rev. 2011;63:209–20.
Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–211.
Kwon IK, Kidoaki S, Matsuda T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: structural characteristics, mechanical properties and cell adhesion potential. Biomaterials. 2005;26:3929–39.
Beachley V, Wen X. Polymer nanofibrous structures: fabrication, biofunctionalization, and cell interactions. Prog Polym Sci. 2010;35:868–92.
Venugopal J, Low S, Choon AT, Ramakrishna S. Interaction of cells and nanofiber scaffolds in tissue engineering. J Biomed Mater Res B Appl Biomater. 2008;84:34–48.
Gunn J, Zhang M. Polyblend nanofibers for biomedical applications: perspectives and challenges. Trends Biotechnol. 2010;28:189–97.
Dew L, English WR, Ortega I, Claeyssens F, MacNeil S. Fabrication of biodegradable synthetic vascular networks and their use as a model of angiogenesis. Cells Tissues Organs. 2016;202:319–28.
Dejana E, Colella S, Languino LR, Balconi G, Corbascio GC, Marchisio PC. Fibrinogen induces adhesion, spreading, and microfilament organization of human endothelial cells in vitro. J Cell Biol. 1987;104:1403–11.
Young WC, Herman IM. Extracellular matrix modulation of endothelial cell shape and motility following injury in vitro. J Cell Sci. 1985;73:19–32.
Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–107.
Schor AM, Schor SL, Allen TD. Effects of culture conditions on the proliferation, morphology and migration of bovine aortic endothelial cells. J Cell Sci. 1983;62:267–85.
Anderson DE, Hinds MT. Extracellular matrix production and regulation in micropatterned endothelial cells. Biochem Biophys Res Commun. 2012;427:159–64.
Hughes CC. Endothelial-stromal interactions in angiogenesis. Curr Opin Hematol. 2008;15:204–9.
Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55:261–8.
Ma Z, He W, Yong T, Ramakrishna S. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Eng. 2005;11:1149–58.
Vleggeert-Lankamp CL, Pêgo AP, Lakke EA, Deenen M, Marani E, Thomeer RT. Adhesion and proliferation of human Schwann cells on adhesive coatings. Biomaterials. 2004;25:2741–51.
Dew L, MacNeil S, Chong CK. Vascularization strategies for tissue engineers. Regen Med. 2015;10:211–24.
Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34.
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71.
Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A. 2008;105:7738–43.
Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA, et al. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–80.
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8.
Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis lymphangiogenesis, vascular fenestrations, and permeability. Circ Res. 2004;94:664–70.
Cheng SY, Nagane M, Huang HS, Cavenee WK. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Sci U S A. 1997;94:12081–7.
Oka N, Soeda A, Inagaki A, Onodera M, Maruyama H, Hara A, et al. VEGF promotes tumorigenesis and angiogenesis of human glioblastoma stem cells. Biochem Biophys Res Commun. 2007;360:553–9.
Laschke MW, Menger MD. Prevascularization in tissue engineering: current concepts and future directions. Biotechnol Adv. 2016;34:112–21.
Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300–11.
Tremblay PL, Hudon V, Berthod F, Germain L, Auger FA. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am J Transplant. 2005;5:1002–10.
Nör JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453–63.
Unger RE, Ghanaati S, Orth C, Sartoris A, Barbeck M, Halstenberg S, et al. The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials. 2010;31:6959–67.
Hadjizadeh A, Doillon CJ. Directional migration of endothelial cells towards angiogenesis using polymer fibres in a 3D co-culture system. J Tissue Eng Regen Med. 2010;4:524–31.
Valdes TI, Kreutzer D, Moussy F. The chick chorioallantoic membrane as a novel in vivo model for the testing of biomaterials. J Biomed Mater Res. 2002;62:273–82.
Lloyd-Griffith C, McFadden TM, Duffy GP, Unger RE, Kirkpatrick CJ, O’Brien FJ. The pre-vascularisation of a collagen-chondroitin sulphate scaffold using human amniotic fluid-derived stem cells to enhance and stabilise endothelial cell-mediated vessel formation. Acta Biomater. 2015;26:263–73.
Kwon YS, Kim JC. Inhibition of corneal neovascularization by rapamycin. Exp Mol Med. 2006;38:173–9.
Shima DT, Adamis AP, Ferrara N, Yeo KT, Yeo TK, Allende R, et al. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med. 1995;1:182–93.
Wu J, Wu Z, Xue Z, Li H, Liu J. PHBV/bioglass composite scaffolds with co-cultures of endothelial cells and bone marrow stromal cells improve vascularization and osteogenesis for bone tissue engineering. RSC Adv. 2017;7:22197–207.
Sorrell JM, Baber MA, Caplan AI. A self-assembled fibroblast-endothelial cell co-culture system that supports in vitro vasculogenesis by both human umbilical vein endothelial cells and human dermal microvascular endothelial cells. Cells Tissues Organs. 2007;186:157–68.
Bishop ET, Bell GT, Bloor S, Broom IJ, Hendry NF, Wheatley DN. An in vitro model of angiogenesis: basic features. Angiogenesis. 1999;3:335–44.
Black AF, Berthod F, L’heureux N, Germain L, Auger FA. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998;12:1331–40.
Hudon V, Berthod F, Black AF, Damour O, Germain L, Auger FA. A tissue-engineered endothelialized dermis to study the modulation of angiogenic and angiostatic molecules on capillary-like tube formation in vitro. Br J Dermatol. 2003;148:1094–104.
Griffith CK, George SC. The effect of hypoxia on in vitro prevascularization of a thick soft tissue. Tissue Eng Part A. 2009;15:2423–34.
Pham QP, Kasper FK, Mistry AS, Sharma U, Yasko AW, Jansen JA, et al. Analysis of the osteoinductive capacity and angiogenicity of an in vitro generated extracellular matrix. J Biomed Mater Res Part A. 2009;88:295–303.
Acknowledgement
The authors are grateful to the Turkish Ministry of National Education for the funding of Ph.D. award to Serkan Dikici.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
Ethical approval for the tissue acquisition was granted by the National Research Ethics Service (NRES) Committee Yorkshire and The Humber–Sheffield (REC Ref.: 15/YH/0177, REC opinion date: 03/06/2015).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dikici, S., Claeyssens, F. & MacNeil, S. Pre-Seeding of Simple Electrospun Scaffolds with a Combination of Endothelial Cells and Fibroblasts Strongly Promotes Angiogenesis. Tissue Eng Regen Med 17, 445–458 (2020). https://doi.org/10.1007/s13770-020-00263-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00263-7