Abstract
Background
A benchmarking survey was conducted by the pharmaceutical collaboration for transparent Medical Information (phactMI™) consortium, with the goal to capture insights from the 27 member companies and their medical information organizations.
Methods
phactMI™ Benchmarking Committee delivered an electronic survey to 27 United States (US) member companies’ Medical Information (MI) departments between December 12, 2017 and February 20, 2018. The survey consisted of approximately 300 questions, divided into 9 topics which included multiple choice and open-ended questions about the following categories: medical information support provided based on different product lifecycles, key metrics measured to assess department performance, and other key services provided by medical information groups.
Results
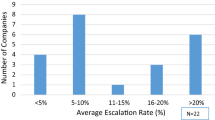
The extent of MI product support varied across the lifecycle of a product. Most companies provided MI product support throughout the product lifecycle starting from investigational all the way through mature; however, the extent of activities varied. The top key performance indicators (KPIs) that were reported to senior leadership included response turnaround time (59%) and inquiry volume (48%). Among the 27 companies, 85% noted using customer satisfaction surveys administered via links within written response documents and 52% verbally via the company's call center. Other services with which MI groups noted most involvement included congress booth support (100%), insights/metric reporting (96%), and training the sales force on the MI function (74%). Additional services included payor support clinical pathway submissions (22%), presenting at advisory boards (22%), and competitive intelligence (26%).
Conclusion
The results of this survey provide pharmaceutical MI groups with opportunities to consider services or activities that could enhance the support these groups provide to their customers and business partners.






Similar content being viewed by others
References
Guillot P, Fung SM. Pharmaceutical medical information contact centers: Results of three benchmarking surveys. Drug Inf J. 2010;44:569–79.
HCP Access to accurate, evidence-based information online is more important than ever. 2017. https://www.lifescienceleader.com/doc/hcp-access-to-accurate-evidence-based-information-online-is-more-important-than-ever-0001.
phactMI: Pharma Collaboration for Transparent Medical Information website. https://www.phactmi.org/PortalAboutUs.
Cadogan A, Fung SM. The changing roles of medical communications professionals: evolution of the core curriculum. Drug Inf J. 2009;43:673–84.
Bordoloi P, et al. Medical information services: how are we trending? Therapeutic Innov Regul Sci. 2014;48:NP5–NP21.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, M., Jindia, L., Fung, S. et al. Pharma Collaboration for Transparent Medical Information (phactMI™) Benchmark Study: Trends, Drivers, and Value of Product Support Activities, Key Performance Indicators, and Other Medical Information Services: Insights from a Survey of 27 US Pharmaceutical Medical Information Departments. Ther Innov Regul Sci 54, 1275–1281 (2020). https://doi.org/10.1007/s43441-020-00162-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-020-00162-y