Abstract
Introduction
Improvement of cognitive function may be desirable for healthy individuals and clinically beneficial for those with cognitive impairment such as from Alzheimer’s disease (AD) or mild cognitive impairment (MCI). The aim of this systematic review is to investigate the cognitive effects of oral saffron intake, in patients with MCI/AD and/or in non-demented individuals, by following the PRISMA guidelines.
Methods
We performed a literature search on MedLine, Cochrane library, and ClinicalTrials.gov to identify randomized controlled trials (RCTs) investigating the effects of oral saffron administration in patients with MCI/AD and/or in non-demented individuals.
Results
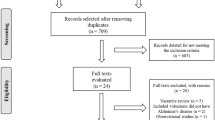
Five studies (enrolling 325 individuals) met our inclusion criteria. Four studies included patients with MCI/AD, and one study included cognitively normal individuals. Saffron was well-tolerated in all groups. Regarding cognitively impaired patients, scores on Alzheimer’s Disease Assessment Scale-cognitive subscale or Mini mental state examination were significantly better when saffron was compared with placebo and did not differ significantly when saffron was compared with donepezil or memantine. Saffron effects on functional status were similar with its effects on cognition.
Conclusions
Saffron was shown to be equally effective to common symptomatic drugs for MCI/AD and resulted in no difference in the incidence of side effects, when compared with placebo or drugs. The promising results should be seen cautiously, since the evidence was derived from studies with potentially high risk of bias (ROB). RCTs with larger sample sizes and low ROB are required to definitively assess the potential role of saffron as an MCI/AD treatment.



Similar content being viewed by others
References
Keuck L (2017) Slicing the cortex to study mental illness: Alois Alzheimer’s pictures of equivalence. Prog Brain Res 233:25–51
Fiest KM, Roberts JI, Maxwell CJ, Hogan DB, Smith EE, Frolkis A, Cohen A, Kirk A, Pearson D, Pringsheim T, Venegas-Torres A, Jetté N (2016) The prevalence and incidence of dementia due to Alzheimer’s disease: a systematic review and meta-analysis. Can J Neurol Sci 43(Suppl 1):S51–S82
Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B (2001) Current concepts in mild cognitive impairment. Arch Neurol 58(12):1985–1992
Becker RE, Kapogiannis D, Greig NH (2018) Does traumatic brain injury hold the key to the Alzheimer’s disease puzzle? Alzheimers Dement 14(4):431–443
Gauthier S, Schlaefke S (2014) Efficacy and tolerability of Ginkgo biloba extract EGb 761(R) in dementia: a systematic review and meta-analysis of randomized placebo-controlled trials. Clin Interv Aging 9:2065–2077
Yang M, Xu DD, Zhang Y, Liu X, Hoeven R, Cho WC (2014) A systematic review on natural medicines for the prevention and treatment of Alzheimer’s disease with meta-analyses of intervention effect of ginkgo. Am J Chin Med 42(3):505–521
Enderami A, Zarghami M, Darvishi-Khezri H (2018) The effects and potential mechanisms of folic acid on cognitive function: a comprehensive review. Neurol Sci 39(10):1667–1675
Chen N, Yang M, Zhou M, Xiao J, Guo J, He L (2017) L-carnitine for cognitive enhancement in people without cognitive impairment. Cochrane Database Syst Rev 3:CD009374
Avgerinos KI, Spyrou N, Bougioukas KI, Kapogiannis D (2018) Effects of creatine supplementation on cognitive function of healthy individuals: a systematic review of randomized controlled trials. Exp Gerontol 108:166–173
Bathaie SZ, Mousavi SZ (2010) New applications and mechanisms of action of saffron and its important ingredients. Crit Rev Food Sci Nutr 50(8):761–786
Christodoulou E, Kadoglou NP, Kostomitsopoulos N, Valsami G (2015) Saffron: a natural product with potential pharmaceutical applications. J Pharm Pharmacol 67(12):1634–1649
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 343:d5928
Akhondzadeh S, Sabet MS, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, Hejazi SS, Yousefi MH, Alimardani R, Jamshidi A, Zare F, Moradi A (2010) Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: a 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther 35(5):581–588
Akhondzadeh S, Shafiee Sabet M, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, Hejazi SS, Yousefi MH, Alimardani R, Jamshidi A, Rezazadeh SA, Yousefi A, Zare F, Moradi A, Vossoughi A (2010) A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology 207(4):637–643
Farokhnia M, Shafiee Sabet M, Iranpour N, Gougol A, Yekehtaz H, Alimardani R, Farsad F, Kamalipour M, Akhondzadeh S (2014) Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: a double-blind randomized clinical trial. Hum Psychopharmacol 29(4):351–359
Tsolaki M, Karathanasi E, Lazarou I, Dovas K, Verykouki E, Karacostas A et al (2016) Efficacy and safety of Crocus sativus L. in patients with mild cognitive impairment: one year single-blind randomized, with parallel groups, clinical trial. J Alzheimers Dis 54(1):129–133
Moazen-Zadeh E, Abbasi SH, Safi-Aghdam H, Shahmansouri N, Arjmandi-Beglar A, Hajhosseinn Talasaz A, Salehiomran A, Forghani S, Akhondzadeh S (2018) Effects of saffron on cognition, anxiety, and depression in patients undergoing coronary artery bypass grafting: a randomized double-blind placebo-controlled trial. J Altern Complement Med 24(4):361–368
Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, van Dyck CH, Aisen PS (2011) A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 77(6):556–563
Group ADC, Bentham P, Gray R, Sellwood E, Hills R, Crome P et al (2008) Aspirin in Alzheimer’s disease (AD2000): a randomised open-label trial. Lancet Neurol 7(1):41–49
Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A et al (2006) Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol 63(10):1402–1408
Modabbernia A, Akhondzadeh S (2013) Saffron, passionflower, valerian and sage for mental health. Psychiatr Clin North Am 36(1):85–91
Sarris J (2007) Herbal medicines in the treatment of psychiatric disorders: a systematic review. Phytother Res 21(8):703–716
Mattson MP (2008) Hormesis defined. Ageing Res Rev 7(1):1–7
Mattson MP (2008) Dietary factors, hormesis and health. Ageing Res Rev 7(1):43–48
Calabrese V, Cornelius C, Trovato A, Cavallaro M, Mancuso C, Di Rienzo L et al (2010) The hormetic role of dietary antioxidants in free radical-related diseases. Curr Pharm Des 16(7):877–883
Premkumar K, Thirunavukkarasu C, Abraham SK, Santhiya ST, Ramesh A (2006) Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum Exp Toxicol 25(2):79–84
Premkumar K, Abraham SK, Santhiya ST, Ramesh A (2003) Protective effects of saffron (Crocus sativus Linn.) on genotoxins-induced oxidative stress in Swiss albino mice. Phytother Res 17(6):614–617
Berger F, Hensel A, Nieber K (2011) Saffron extract and trans-crocetin inhibit glutamatergic synaptic transmission in rat cortical brain slices. Neuroscience. 180:238–247
Geromichalos GD, Lamari FN, Papandreou MA, Trafalis DT, Margarity M, Papageorgiou A, Sinakos Z (2012) Saffron as a source of novel acetylcholinesterase inhibitors: molecular docking and in vitro enzymatic studies. J Agric Food Chem 60(24):6131–6138
Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, Lamari FN (2006) Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem 54(23):8762–8768
Montoro P, Maldini M, Luciani L, Tuberoso CI, Congiu F, Pizza C (2012) Radical scavenging activity and LC-MS metabolic profiling of petals, stamens, and flowers of Crocus sativus L. J Food Sci 77(8):C893–C900
Lyketsos CG, Olin J (2002) Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry 52(3):243–252
Scaricamazza E, Colonna I, Sancesario GM, Assogna F, Orfei MD, Franchini F, Sancesario G, Mercuri NB, Liguori C (2019) Neuropsychiatric symptoms differently affect mild cognitive impairment and Alzheimer’s disease patients: a retrospective observational study. Neurol Sci 40:1377–1382
Starkstein SE, Mizrahi R, Power BD (2008) Depression in Alzheimer’s disease: phenomenology, clinical correlates and treatment. Int Rev Psychiatry 20(4):382–388
Spalletta G, Caltagirone C, Padovani A, Sorbi S, Attar M, Colombo D, Cravello L, on behalf of the E V O L U T I O N study Working Group (2014) Cognitive and affective changes in mild to moderate Alzheimer’s disease patients undergoing switch of cholinesterase inhibitors: a 6-month observational study. PLoS One 9(2):e89216
Spalletta G, Gianni W, Giubilei F, Casini AR, Sancesario G, Caltagirone C, Cravello L (2013) Rivastigmine patch ameliorates depression in mild AD: preliminary evidence from a 6-month open-label observational study. Alzheimer Dis Assoc Disord 27(3):289–291
Banerjee S, Hellier J, Dewey M, Romeo R, Ballard C, Baldwin R, Bentham P, Fox C, Holmes C, Katona C, Knapp M, Lawton C, Lindesay J, Livingston G, McCrae N, Moniz-Cook E, Murray J, Nurock S, Orrell M, O'Brien J, Poppe M, Thomas A, Walwyn R, Wilson K, Burns A (2011) Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet. 378(9789):403–411
Dudas R, Malouf R, McCleery J, Dening T (2018) Antidepressants for treating depression in dementia. Cochrane Database Syst Rev 8:CD003944
Akhondzadeh S, Fallah-Pour H, Afkham K, Jamshidi AH, Khalighi-Cigaroudi F (2004) Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816]. BMC Complement Altern Med 4:12
Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, Fallah-Pour H, Jamshidi AH, Khani M (2005) Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res 19(2):148–151
Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi AH (2005) Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol 97(2):281–284
Funding
The present study was supported in part by the Intramural Program of the National Institute on Aging, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
None.
Informed consent
There was no need for informed consent before conducting the present study, since it was a systematic review of literature.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avgerinos, K.I., Vrysis, C., Chaitidis, N. et al. Effects of saffron (Crocus sativus L.) on cognitive function. A systematic review of RCTs. Neurol Sci 41, 2747–2754 (2020). https://doi.org/10.1007/s10072-020-04427-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04427-0