Abstract
Background/objective
Multivariable prognostic scores play an important role for clinical decision-making, information giving to patients/relatives, benchmarking and guiding clinical trial design. Coagulopathy has been implicated on trauma and critical care outcomes, but few studies have evaluated its role on traumatic brain injury (TBI) outcomes. Our objective was to verify the incremental prognostic value of routine coagulopathy parameters in addition to the CRASH-CT score to predict 14-day mortality in TBI patients.
Methods
This is a prospective cohort of consecutive TBI patients admitted to a tertiary university hospital Trauma intensive care unit (ICU) from March/2012 to January/2015. The prognostic performance of the coagulation parameters platelet count, prothrombin time (international normalized ratio, INR) and activated partial thromboplastin time (aPTT) ratio was assessed through logistic regression adjusted for the original CRASH-CT score. A new model, CRASH-CT-Coag, was created and its calibration (Brier scores and Hosmer–Lemeshow (H–L) test), discrimination [area under the receiver operating characteristic curve (AUC-ROC) and the integrated discrimination improvement (IDI)] and clinical utility (net reclassification index) were compared to the original CRASH-CT score.
Results
A total 517 patients were included (median age 39 years, 85.1% male, median admission glasgow coma scale 8, neurosurgery on 44.9%). The 14-day mortality observed and predicted by the original CRASH-CT was 22.8% and 26.2%, respectively. Platelet count < 100,000/mm3, INR > 1.2 and aPTT ratio > 1.2 were present on 11.3%, 65.0% and 27.2%, respectively, (at least one of these was altered on 70.6%). All three variables maintained statistical significance after adjustment for the CRASH-CT score. The CRASH-CT-Coag score outperformed the original score on calibration (brier scores 0.122 ± 0.216 vs 0.132 ± 0.202, mean difference 0.010, 95% CI 0.005–0.019, p = 0.036, respectively) and discrimination (AUC-ROC 0.854 ± 0.020 vs 0.813 ± 0.024, p = 0.014; IDI 5.0%, 95% CI 1.3–11.0%). Both scores showed the satisfactory H–L test results. The net reclassification index favored the new model. Considering the strata of low (< 10%), moderate (10–30%) and high (> 30%) risk of death, the CRASH-CT-Coag model yielded a global net correct reclassification of 22.9% (95% CI 3.8–43.4%).
Conclusions
The addition of early markers of coagulopathy—platelet count, INR and aPTT ratio—to the CRASH-CT score increased its accuracy. Additional studies are required to externally validate this finding and further investigate the coagulopathy role on TBI outcomes.
Similar content being viewed by others
Introduction
Multivariable prognostic scores are the key not only for therapeutic management and clinical decision-making, but also to reliably inform patients and relatives, to guide trials design and for benchmarking the quality of care by comparing observed and expected outcomes [1]. The corticosteroid randomization after significant head injury (CRASH) score is one of the main validated prognostic models for traumatic brain injury (TBI) [2, 3]. These and other TBI prognostic scores have underevaluated coagulopathy markers, which have been implicated on trauma and critical care patients’ outcomes.
Few studies have evaluated the role of coagulopathy markers on TBI outcomes, and most of them have not performed an analysis adjusted for already-validated prognostic scores [4, 5]. Only one study has formally evaluated the incremental prognostic value of coagulopathy in addition to a well-grounded prognostic score [6]. Indeed, coagulopathy improved the discrimination ability of the model by increasing its area under the receiver operating characteristic curve (AUC-ROC); however, the calibration and clinical utility (net reclassification index, NRI) of the new model were not fully assessed.
Here, we aimed to verify the incremental prognostic value (discrimination, calibration and clinical utility) of routine hospital admission coagulopathy parameters (platelet count, prothrombin time and activated partial thromboplastin time) in addition to the CRASH score to predict 14-day mortality outcome in TBI patients.
Materials and Methods
This is a prospective cohort that included consecutive TBI patients admitted to a trauma intensive care unit (ICU) of a tertiary university hospital (Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo—HC/FMUSP) from March 2012 to January 2015. Patients under 14 years old, victims of penetrating TBI or admitted from another ICU were excluded. Chronic subdural hematomas were also excluded.
Clinical, laboratory and radiological data were registered, as well as the primary outcome, 14-day death. Clinical predictors were defined as recommended by the brain trauma foundation and similar to the CRASH study protocol, including the definitions of major extracranial injury and head computed tomography (CT) alterations [2, 7]. Imaging (head computed tomography) was reviewed by a neurosurgeon blinded to the clinical data. Laboratory variables were registered at hospital admission, thus prior to the primary outcome. The percentages of missing data were: pupil reactivity at admission 5.8%; admission Glasgow coma scale (GCS) 5.0%; extracranial lesion at admission 1.2%; and age 1.2%. Since the original CRASH-CT score exact risk probability for each patient was crucial for the study objective and analysis, we decided not to execute multiple imputation and to perform a complete-case analysis.
The study protocol was approved by the local ethics committee (Comissão de Análise de Projetos de Pesquisa—CAPPesq, HC/FMUSP, protocol number 00119/10).
Statistical Analysis
Categorical variables were described as absolute and relative frequencies and compared through the χ2 test or the Fisher exact test, as appropriate. Continuous variables distributions were evaluated by skewness and kurtosis values as well as graphical methods. Those with normal distributions were described as means and standard deviations and compared through the independent samples Student T test. The ones with non-normal distributions were described as medians and quartiles and compared through the Mann–Whitney test.
The performance of the coagulation parameters as predictors of 14-day death was assessed through logistic regression analysis with adjustment for the original CRASH-CT score. Platelet count, prothrombin time (international normalized ratio, INR) and activated partial thromboplastin time (aPTT, ratio) were included as binary variables for the sake of clinical utility: Platelet count was deemed low if < 100,000/mm3; INR was considered altered if > 1.2; and aPTT was considered abnormal if its ratio was > 1.2. Only cases with complete data for the CRASH-CT score calculations were included in the respective models. Data regarding pre-hospital hypoxia or admission glucose was missing for almost half of the cohort, so we were not able to perform the same analysis with adjustment to the extended IMPACT model.
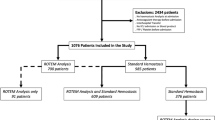
A new model, CRASH-CT-Coag, was created after the above-cited logistic regression adjustment and, thus, included the original CRASH-CT score, platelet count, INR and aPTT ratio. The latter covariates, the coagulation parameters, were included as binary variables (altered or normal). The CRASH-CT-Coag accuracy was compared to the original CRASH-CT score. The calibration was evaluated by the Hosmer–Lemeshow goodness-of-fit test, which compares the predicted and observed death probabilities in each risk decile, and by the Brier scores, a measure of the mean squared deviation between the predicted death probabilities and the observed outcomes. The discrimination was evaluated by the respective areas under the receiver operating characteristic curve (AUC-ROC), which were compared through the DeLong method, and by the integrated discrimination improvement (IDI), a measure of the difference in predicted death probabilities between the survivors and dead subjects. The clinical utility of the new prognostic model was evaluated by the net reclassification index (NRI). The NRI calculation had the assumption of the following death risk stratification: low (< 10%), moderate (10–30%) and high (> 30%) risk. The decision for this death risk stratification was empirical, but other stratification schemes were explored and generally lead to similar conclusions. Bootstrap sampling (1000 samples) was employed for the calculation of point estimates, standard errors and confidence intervals (CI). The statistical analysis flowchart is depicted in Fig. 1.
All tests were two-tailed, and final p values < 0.05 were considered statistically significant. These were the softwares employed for the analysis: R (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria), SPSS (IBM Corp. SPSS Statistics para Windows, versão 24.0. Armonk, NY) and MedCalc Statistical Software (MedCalc Software bvba, versão 15.2.0.0. Ostend, Belgium). The transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) statement was followed for this study report.
Results
A total of 517 patients were included, with a median age of 39 (quartiles 27–52) years and 85.1% male. The three most common trauma mechanisms were fall from height (30.1%), fall from own height (26.1%) and motorcycle accident (19.5%). The median admission GCS was 8 (6–13), and pupil reactivity was absent/sluggish unilaterally on 12.9% and bilaterally on 5.9%. Major extracranial lesion was present on 54.4%, and neurosurgery was performed on 44.9% of the patients. These data and the head CT findings are summarized in Table 1. The mean 14-day death probability predicted by the original CRASH-CT score was 26.2%, and the in-hospital death probability predicted by the SAPS3 score was 26.4%. The observed 14-day and in-hospital death were 22.8% and 30.9%, respectively. The CRASH-CT score was available for 452 patients, with similar characteristics compared to the full cohort.
Thrombocytopenia, INR > 1.2 and aPTT > 1.2 were present on 11.3%, 65.0% and 27.2%, respectively. At least one of these parameters was altered on 70.6%. The univariate analyses for the coagulation parameters are presented in Table 2. Platelet count, prothrombin time and aPTT levels were associated with 14-day outcome. Platelet count was lower, and thrombocytopenia (< 100,000/mm3) was more common on the subgroup that evolved to death. Similarly, INR and aPTT were higher and more commonly altered (> 1.2) on this subgroup. These results were the same for the subset with the CRASH-CT score available (data not shown).
The three coagulation parameters of interest were included as binary variables (normal or altered) on the multivariable logistic regression analyses. Platelet count below 100,000/mm3 (OR 2.45, 95% CI 1.14–5.25, p = 0.021), INR above 1.2 (OR 2.03, 95% CI 1.05–3.94, p = 0.036) and aPTT ratio above 1.2 (OR 2.50, 95% CI 1.41–4.48, p = 0.002) maintained statistical significance after adjustment for the CRASH-CT score. More details of the multivariable model are presented in Table 3.
As stated on the methods section, a new model, CRASH-CT-Coag, was created and compared to the original CRASH-CT model regarding calibration, discrimination and clinical utility. The mean 14-day death probability predicted by the CRASH-CT-Coag was 22.1% (± 12.2). The CRASH-CT-Coag score outperformed the CRASH-CT score on calibration as assessed by the Brier scores (0.122 ± 0.216 vs 0.132 ± 0.202, mean difference 0.010, 95%CI 0.005–0.019, p = 0.036, respectively). This indicates that the new model leads to overall smaller differences between the predicted death probabilities and the observed outcomes (Fig. 2, panel A). Both scores showed the satisfactory results by the H–L test (x2 = 12.8, p = 0.118 and x2 = 11.0, p = 0.201, respectively) (Fig. 2, panels C and D).
Panel A: Observed outcomes and predicted death probability according to each model. The CRASH-CT-Coag score outperformed the CRASH-CT score on calibration (Brier scores 0.122 ± 0.216 vs 0.132 ± 0.202, mean difference 0.010, 95% CI 0.005–0.019, p = 0.036). This indicates that the new model leads to overall smaller differences between the predicted death probabilities and the observed outcomes. Also, the mean risk difference on predicted death probabilities for those who survived and those who did not was higher for the CRASH-CT-Coag model (29.4%, 95% CI 21.1–39.0 vs 24.4%, 95% CI 16.3–33.2, p < 0.001). This implies an integrated discrimination improvement (IDI) of 5.0% (95% CI 1.3–11.0%), which is the additional difference on predicted death probabilities between the survivors and the dead subjects; Panel B: Receiver operating characteristic (ROC) curve analysis. The CRASH-CT-Coag score also had a better discrimination. The AUC-ROC for the new model was 0.854 ± 0.020 vs 0.813 ± 0.024 for the original one (p = 0.014); Panels C and D: Observed and predicted death probabilities by estimated risk deciles. Both scores showed satisfactory results by the H–L test (x2 = 12.8, p = 0.118 and x2 = 11.0, p = 0.201, respectively)
The CRASH-CT-Coag score also had a better discrimination. The AUC-ROC for the new model was 0.854 ± 0.020 versus 0.813 ± 0.024 for the original one (p = 0.014) (Fig. 2, panel B). Also, the mean risk difference on predicted death probabilities for those who survived and those who did not was higher for the CRASH-CT-Coag model (29.4%, 95% CI 21.1–39.0 vs 24.4%, 95% CI 16.3–33.2, p < 0.001). This implies an IDI of 5.0% (95% CI 1.3–11.0%), which is the additional difference on predicted death probabilities between the survivors and the dead subjects (Fig. 2, panel A).
Considering the strata of low (< 10%), moderate (10–30%) and high (> 30%) risk of death, the CRASH-CT-Coag model, compared to the original CRASH-CT, would yield a global net correct reclassification of 22.9% (95% CI 3.8–43.4%) (Table 4). Among the survivors, the NRI to a lower risk stratum was 8.5% (95% CI 0.3–26.9%). Among the dead ones, the NRI to a higher risk stratum was 14.4% (95% CI 0.9–22.6%). In other words, the CRASH-CT-Coag score had a better performance regardless of the outcome. In Fig. 3, the predicted death probabilities by the new model were plotted against the probability predicted by the original CRASH-CT model. The new predicted death probability strata were higher on the red area, the same on the white area and lower on the green area. Thus, the ideal scenario would be for the dead subjects to be on the red area and the survivors on the green area. The four discernible groups visually shown in Fig. 3 reflect the difference between the models underevaluation, which is due to the three dichotomous variables (normal or altered platelet count, INR and aPTT ratio, which OR are similarly around 2–2.5).
Predicted death probabilities for the original and new models according to risk strata and 14-day outcome. The new predicted death probability strata are higher on the red area, the same on the white area and lower on the green area. The four discernible groups visually seen reflect the difference between the models under evaluation, which is due to the three dichotomous variables (normal or altered platelet count, INR and aPTT ratio, which odds ratio are similarly around 2–2.5)
Case examples are presented in Table 5 to illustrate how the adjusted model could impact the predicted outcome probability.
Discussion
Coagulopathy is common after TBI, and its reported incidence may vary from 10% to more than 90%, depending on the definition, for which there is no consensus yet [8, 9]. This disorder may start from minutes to hours after TBI and, although most develop it at the first 24 h, it may occur up to the fifth-day post-injury and extend for more than 72 h in 30% of the cases [10]. It seems to be associated with the severity of the injury, rather than its location [11, 12]. However, coagulopathy itself exerts an independent effect on mortality after TBI [10, 12, 13]. Thus, the hypothesis that coagulopathy markers would improve the available TBI scores is sound.
We have shown that coagulopathy markers may increase the prognostic accuracy of the CRASH-CT score, although it performed quite well in our population. Each altered parameter, platelet count, INR or aPTT ratio, more than doubled the odds of death. Different methodologies were used to assess all components of accuracy and were consistent on showing the new model superiority regarding discrimination, calibration and clinical utility. The parameters analyzed are inexpensive and usually routinely measured on trauma and intensive care patients. Also, the cutoffs used for the dichotomous classification (altered or normal) are internationally standardized and well-grounded on clinical practice—rather than artificial cutoffs to maximize statistical significance. To our knowledge, this is the one of the most comprehensive evaluations of routine coagulopathy markers for TBI prognostication. Yuan et al. [14] have also demonstrated that the addition of the coagulation test results (INR and aPTT) to a newly developed multivariable model including age, neurological examination, imaging and glucose can improve its predictive ability, but they have not compared it against a previously validated score (as the CRASH or the IMPACT models).
Some critical care scores do include some of these coagulopathy indicators as risk markers or worse outcome predictors. Moreover, although the original CRASH score did not evaluate laboratory variables, the international mission for prognosis and analysis of clinical trials in traumatic brain injury (IMPACT) original study analysis—another well-grounded TBI prognostic tool—was significant for platelets and INR as predictors of outcome [15]. Ultimately, platelets and INR were not included in the final IMPACT score because these measures were available on less than 20% and 10%, respectively, of the total study sample. The aPTT values were not analyzed in the IMPACT study.
The above relates to the first limitation to be noted in our study. The IMPACT extended model includes variables that were not fully addressed by the protocol of this study and hypoxia and glucose were not available at admission for almost half of the sample. Thus, we could not perform a similar analysis with adjustment for the IMPACT extended model. However, the IMPACT model was developed based on patients from high-income countries, whereas the CRASH data were mainly collected from low- and middle-income ones. It has been shown that the IMPACT model may not fit the CRASH data so well [16]. Even though we only analyzed the early 14-day vital status, this is a trustworthy outcome and robust against rehabilitation capacity shortages, which is still a problem in middle-income countries and impacts the long-term clinical results. Another constraint is the literature heterogeneity regarding the definition of coagulopathy. For instance, coagulopathy incidence variation has been attributed to differences in study designs, injury diversity, different time points for testing of coagulation parameters and the absence of a universally recognized definition of coagulopathy [8, 9]. As we said, the selected cutoffs for platelet count, INR and aPTT ratio are standard and common on clinical practice, but we recognize that any dichotomization of a continuous variable may carry some bias and may be criticized. The dynamic nature of TBI poses additional challenges on that matter. Lastly, although the incremental prognostic value of coagulopathy was consistent across different methods of analysis, an external validation of this proposal of prognostic model extension is crucial for its endorsement and clinical application. Additional tests for the evaluation of the coagulation system—enzymatic processes, fibrinolysis and platelets function—are becoming more widely available (e.g., fibrinogen and thromboelastography), and these may gain further relevance on coagulopathy management and outcome prognosis.
Opportunities for updating the CRASH and IMPACT models may be brought in the near future by noteworthy studies, including the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER‐TBI) study, Transforming Research and Clinical Knowledge in TBI (TRACK‐TBI) dataset and Collaborative REsearch on ACute Traumatic Brain Injury in intensiVe Care Medicine in Europe (CREACTIVE) [17,18,19].
Conclusion
The addition of platelet count, INR and aPTT, interpreted as early markers of coagulopathy, to the CRASH-CT score increased its accuracy, with better discrimination, calibration and clinical utility. Additional studies are required to externally validate this finding and further investigate its implications for TBI management. Moreover, this result underscores the need for further investigation of the coagulopathy role on TBI outcomes.
Abbreviations
- aPTT:
-
Activated partial thromboplastin time
- AUC-ROC:
-
Area under the receiver operating characteristic curve
- CENTER‐TBI:
-
Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury study
- CREACTIVE:
-
Collaborative REsearch on ACute Traumatic Brain Injury in intensiVe Care Medicine in Europe
- CRASH:
-
Corticosteroid randomization after significant head injury
- CT:
-
Computed tomography
- GCS:
-
Glasgow coma scale
- HC/FMUSP:
-
Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo
- ICU:
-
Intensive care unit
- IDI:
-
Integrated discrimination improvement
- IMPACT:
-
International mission for prognosis and analysis of clinical trials in traumatic brain injury
- INR:
-
International normalized ratio
- NRI:
-
Net reclassification index
- TBI:
-
Traumatic brain injury
- TRACK‐TBI:
-
Transforming research and clinical knowledge in TBI
- TRIPOD:
-
Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis
References
Lingsma HF, Roozenbeek B, Steyerberg EW, Murray GD, Maas AIR. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol. 2010;9(5):543–54.
Crash MRC, Collaborators T. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336(7641):425–9.
Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165 (discussion e165).
Chhabra G, Sharma S, Subramanian A, Agrawal D, Sinha S, Mukhopadhyay AK. Coagulopathy as prognostic marker in acute traumatic brain injury. J Emerg Trauma Shock. 2013;6(3):180–5.
Talving P, Benfield R, Hadjizacharia P, Inaba K, Chan LS, Demetriades D. Coagulopathy in severe traumatic brain injury: a prospective study. J Trauma. 2009;66(1):55–61 (discussion 61-2).
Raj R, Siironen J, Kivisaari R, et al. External validation of the international mission for prognosis and analysis of clinical trials model and the role of markers of coagulation. Neurosurgery. 2013;73(2):305–11 (discussion 311).
Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–S106.
Harhangi BS, Kompanje EJO, Leebeek FWG, Maas AIR. Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien). 2008;150(2):165–75 (discussion 175).
Laroche M, Kutcher ME, Huang MC, Cohen MJ, Manley GT. Coagulopathy after traumatic brain injury. Neurosurgery. 2012;70(6):1334–455.
Lustenberger T, Talving P, Kobayashi L, et al. Time course of coagulopathy in isolated severe traumatic brain injury. Injury. 2010;41(9):924–8.
Genét GF, Johansson PI, Meyer MAS, et al. Trauma-induced coagulopathy: standard coagulation tests, biomarkers of coagulopathy, and endothelial damage in patients with traumatic brain injury. J Neurotrauma. 2013;30(4):301–6.
de Oliveira Manoel AL, Neto AC, Veigas PV, Rizoli S. Traumatic brain injury associated coagulopathy. Neurocrit Care. 2015;22(1):34–44.
Wafaisade A, Lefering R, Tjardes T, et al. Acute coagulopathy in isolated blunt traumatic brain injury. Neurocrit Care. 2010;12(2):211–9.
Yuan Q, Yu J, Wu X, et al. Prognostic value of coagulation tests for in-hospital mortality in patients with traumatic brain injury. Scand J Trauma Resusc Emerg Med. 2018;26(1):3.
Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):329–37.
Gao J, Zheng Z. Development of prognostic models for patients with traumatic brain injury: a systematic review. Int J Clin Exp Med. 2015;8(11):19881–5.
CENTER-TBI. Collaborative European neurotrauma effectiveness research in TBI—ClinicalTrials.gov Identifier: NCT02210221.
TRACK-TBI. Transforming research and clinical knowledge in traumatic brain injury—ClinicalTrials.gov Identifier: NCT02119182.
CREACTIVE. Collaborative REsearch on ACute Traumatic Brain Injury in intensiVe Care Medicine in Europe—ClinicalTrials.gov Identifier: NCT02004080.
Funding
This research was partially funded by the National Council for Scientific and Technological Development (CNPq), Brazil. Drs Solla, Kolias, Hutchinson and Paiva are supported by the NIHR Global Health Research Group on Neurotrauma, which was commissioned by the National Institute for Health Research (NIHR) using UK aid from the UK Government (project 16/137/105). The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. Angelos Kolias is supported by a Clinical Lectureship, School of Clinical Medicine, University of Cambridge and the Royal College of Surgeons of England. Peter Hutchinson is supported by a Research Professorship from the NIHR, the NIHR Cambridge Biomedical Research Centre, a European Union Seventh Framework Program grant (CENTER-TBI; grant no. 602150), and the Royal College of Surgeons of England.
Author information
Authors and Affiliations
Contributions
Drs. Solla, Amorim and Paiva had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Solla, Amorim and Paiva participated in concept and design. Solla, Amorim, Kolias, Hutchinson, Andrade, Teixeira and Paiva contributed in acquisition, analysis or interpretation of data. Solla conducted the statistical analysis and drafted the manuscript. Solla, Amorim, Kolias, Hutchinson, Andrade, Teixeira and Paiva participated in critical revision of the manuscript for important intellectual content. Amorim and Paiva obtained the funding. Paiva, Amorim, Andrade, Kolias and Hutchinson contributed in supervision.
Corresponding author
Ethics declarations
Conflict of interest
Davi J. Fontoura Solla: Dr. Solla reports grants and non-financial support from National Institute for Health Research (NIHR), during the conduct of the study. Robson Luis Oliveira de Amorim: Dr. Amorim reports grants from National Council for Scientific and Technological Development (CNPq), Brazil, during the conduct of the study. Angelos G. Kolias: Dr. Kolias reports grants and non-financial support from National Institute for Health Research (NIHR), grants and non-financial support from School of Clinical Medicine, University of Cambridge, grants and non-financial support from Royal College of Surgeons of England, during the conduct of the study. Peter J. Hutchinson: Dr. Hutchinson reports grants and non-financial support from National Institute for Health Research (NIHR), grants and non-financial support from NIHR Cambridge Biomedical Research Centre, grants and non-financial support from Royal College of Surgeons of England, grants from European Union Seventh Framework Program, during the conduct of the study. Almir Ferreira de Andrade: Dr. Andrade has nothing to disclose. Manoel Jacobsen Teixeira: Dr. Jacobsen-Teixeira has nothing to disclose. Wellingson Silva Paiva: Dr. Paiva reports grants and non-financial support from National Institute for Health Research (NIHR), during the conduct of the study.
Ethical Approval
We confirm the adherence to ethical guidelines—this study protocol was approved by the local ethics committee (Comissão de Análise de Projetos de Pesquisa—CAPPesq, HC/FMUSP, Protocol Number 00119/10). The Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) Statement was followed for this study report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An earlier version of the study was presented orally on the 2018 CNS Congress (“Oral Presentations of the 2018 Annual Meeting of the Congress of Neurological Surgeons”).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Solla, D.J.F., de Amorim, R.L.O., Kolias, A.G. et al. Incremental Prognostic Value of Coagulopathy in Addition to the Crash Score in Traumatic Brain Injury Patients. Neurocrit Care 34, 130–138 (2021). https://doi.org/10.1007/s12028-020-00991-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-00991-7