Abstract
Recent dramatic declines in global malaria burden and mortality can be largely attributed to the large-scale deployment of insecticidal-based measures, namely long-lasting insecticidal nets (LLINs) and indoor residual spraying. However, the sustainability of these gains, and the feasibility of global malaria eradication by 2040, may be affected by increasing insecticide resistance among the Anopheles malaria vector. We employ a new differential-equations based mathematical model, which incorporates the full, weather-dependent mosquito lifecycle, to assess the population-level impact of the large-scale use of LLINs, under different levels of Anopheles pyrethroid insecticide resistance, on malaria transmission dynamics and control in a community. Moreover, we describe the bednet-mosquito interaction using parameters that can be estimated from the large experimental hut trial literature under varying levels of effective pyrethroid resistance. An expression for the basic reproduction number, \({\mathcal {R}}_0\), as a function of population-level bednet coverage, is derived. It is shown, owing to the phenomenon of backward bifurcation, that \({\mathcal {R}}_0\) must be pushed appreciably below 1 to eliminate malaria in endemic areas, potentially complicating eradication efforts. Numerical simulations of the model suggest that, when the baseline \({\mathcal {R}}_0\) is high (corresponding roughly to holoendemic malaria), very high bednet coverage with highly effective nets is necessary to approach conditions for malaria elimination. Further, while >50% bednet coverage is likely sufficient to strongly control or eliminate malaria from areas with a mesoendemic malaria baseline, pyrethroid resistance could undermine control and elimination efforts even in this setting. Our simulations show that pyrethroid resistance in mosquitoes appreciably reduces bednet effectiveness across parameter space. This modeling study also suggests that increasing pre-bloodmeal deterrence of mosquitoes (deterring them from entry into protected homes) actually hampers elimination efforts, as it may focus mosquito biting onto a smaller unprotected host subpopulation. Finally, we observe that temperature affects malaria potential independently of bednet coverage and pyrethroid-resistance levels, with both climate change and pyrethroid resistance posing future threats to malaria control.











Similar content being viewed by others
References
Agusto FB, Gumel AB, Parham PE (2015) Qualitative assessment of the role of temperature variations on malaria transmission dynamics. J Biol Syst 23(4):1–34
Alles HK, Mendis KN, Carter R (1998) Malaria mortality rates in South Asia and in Africa: implications for malaria control. Parasitol Today 14:369–375
Alout H, Roche B, Dabiré RK, Cohuet A (2017) Consequences of insecticide resistance on malaria transmission. PLoS Pathog 13(9):e1006499
Asale A, Getachew Y, Hailesilassie W, Speybroeck N, Duchateau L, Yewhalaw D (2014) Evaluation of the efficacy of DDT indoor residual spraying and long-lasting insecticidal nets against insecticide resistant populations of Anopheles arabiensis Patton (Diptera: Culicidae) from Ethiopia using experimental huts. Parasites Vectors 7(1):131
Ashley EA, White NJ (2014) The duration of Plasmodium falciparum infections. Malar J 13:500
Asidi AN, N’Guessan R, Hutchinson RA, Traorá LM, Carnevale P, Curtis CF (2004) Experimental hut comparisons of nets treated with carbamate or pyrethroid insecticides, washed or unwashed, against pyrethroid-resistant mosquitoes. Med Vet Entomol 18(2):134–140
Asidi AN, N’Guessan R, Koffi AA, Curtis CF, Hougard JM, Chandre F, Rowland MW (2005) Experimental hut evaluation of bednets treated with an organophosphate (chlorpyrifos-methyl) or a pyrethroid (lambdacyhalothrin) alone and in combination against insecticide-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes. Malar J 4(1):25
Barbosa S, Hastings IM (2012) The importance of modeling the spread of insecticide resistance in a heterogeneous environment: the example of adding synergists to bednets. Malar J 11:258
Bayili K, N’do S, Namountougou M, Sanou R, Ouattara A, Dabiré RK, Diabaté AA (2017) Evaluation of efficacy of interceptor\(\textregistered \) G2, a long-lasting insecticide net coated with a mixture of chlorfenapyr and alpha-cypermethrin, against pyrethroid resistant Anopheles gambiae sl in Burkina Faso. Malar J 16(1):190
Bayoh MN (2001) Studies on the development and survival of Anopheles gambiae sensu stricto at various temperatures and relative humidities. Doctoral dissertation. Durham Theses, Durham University. http://etheses.dur.ac.uk/4952/
Bayoh MN, Lindsay SW (2003) Effect of temperature on the development of the aquatic stages of Anopheles gambiae sensu stricto (Diptera: Culicidae). Bull Entomol Res 93:375–381
Bayoh MN, Lindsay SW (2004) Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Med Vet Entomol 18:174–179
Beck-Johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, BjØrnstad ON (2017) The importance of temperature fluctuations in understanding mosquito population dynamics and malaria risk. R Soc Open Sci 4:160969. https://doi.org/10.1098/rsos.160969
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle K, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briët O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CLJ, Smith DL, Hay SI, Cibulskis RE, Gething PW (2015) The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526:207–211
Birget PLG, Koella JC (2015a) A genetic model of the effects of insecticide-treated bed nets on the evolution of insecticide-resistance. Evol Med Public Health 2015:205–215
Birget PLG, Koella JC (2015b) An epidemiological model of the effects of insecticide treated bed nets on malaria transmission. PloS One 10(12):e0144173. https://doi.org/10.1371/journal.pone.0144173
Blayneh K, Gumel AB, Lenhart S, Clayton T (2010) Backward bifurcation analysis and optimal control of West Nile virus. Bull Math Biol 72(4):1006–1028
Briere JF, Pracros P, le Roux AY, Pierre S (1999) A novel rate model of temperature-dependent development for arthropods. Environ Entomol 28:22–29
Brown ZS, Dickinson KL, Kramer RA (2013) Insecticide resistance and malaria vector control: the importance of fitness cost mechanisms in determining economically optimal control trajectories. J Econ Entomol 106(1):366–374
Camara S, Alou LPA, Koffi AA, Clegban YCM, Kabran JP, Koffi FM, Pennetier C (2018) Efficacy of interceptor\(\textregistered \) G2, a new long-lasting insecticidal net against wild pyrethroid-resistant Anopheles gambiae ss from Côte d’Ivoire: a semi-field trial. Parasite 25:42
Carr J (1981) Application of centre manifold theory. Springer, New York
Castillo-Chavez CC, Song B (2004) Dynamical models of tuberculosis and their applications. Math Biosci Eng 1:361–404
Cator LJ, Lynch PA, Read AF, Thomas MB (2012) Do malaria parasites manipulate mosquitoes? Trends Parasitol 28(11):466–470
Chandre F, Darriet F, Duchon S, Finot L, Manguin S, Carnevale P, Guillet P (2000) Modifications of pyrethroid effects associated with kdr mutation in Anopheles gambiae. Med Vet Entomol 14(1):81–88
Charlwood JD, Smith T, Billingsley PF, Takken W, Lyimo EOK, Meuwissen JHET (1997) Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans. Bull Entomol Res 87:445–453
Chitnis N, Smith T, Steketee R (2009) A mathematical model for the dynamics of malaria in mosquitoes feeding on a heterogeneous host population. J Biol Dyn 2(3):259–285
Corbel V, Chandre F, Brengues C, Akogbéto M, Lardeux F, Hougard JM, Guillet P (2004) Dosage-dependent effects of permethrin-treated nets on the behaviour of Anopheles gambiae and the selection of pyrethroid resistance. Malar J 3(1):22
Corbel V, Chabi J, Dabiré RK, Etang J, Nwane P, Pigeon O, Hougard JM (2010) Field efficacy of a new mosaic long-lasting mosquito net (PermaNet\(\textregistered \) 3.0) against pyrethroid-resistant malaria vectors: a multi centre study in Western and Central Africa. Malar J 9(1):113
Detinova TS, Bertram DS, World Health Organization (1962) Age-groupingmethods in diptera of medical importance, with special reference to some vectors of malaria / Detinova TS ; [with] an Annex on the ovary and ovarioles of mosquitos (with glossary) by Bertram DS. World Health Organization. https://apps.who.int/iris/handle/10665/41724
Djènontin A, Alou LPA, Koffi A, Zogo B, Duarte E, N’Guessan R, Pennetier C (2015) Insecticidal and sterilizing effect of Olyset Duo\(\textregistered \), a permethrin and pyriproxyfen mixture net against pyrethroid-susceptible and-resistant strains of Anopheles gambiae ss: a release-recapture assay in experimental huts. Parasite 22:27
Djènontin A, Moiroux N, Bouraïma A, Zogo B, Sidick I, Corbel V, Pennetier C (2018) Field efficacy of a new deltamethrin long lasting insecticidal net (LifeNet\(\textregistered \)) against wild pyrethroid-resistant Anopheles gambiae in Benin. BMC Public Health 18(1):947
Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, White NJ (2009) Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361(5):455–467
Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, Kivaya E (2010) Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376:1647–1657
Eikenberry SE, Gumel AB (2018) Mathematical modeling of climate change and malaria transmission dynamics: a historical review. J Math Biol 77:857–933
Fanello C, Kolaczinski JH, Conway DJ, Carnevale P, Curtis CF (1999) The kdr pyrethroid resistance gene in Anopheles gambiae: tests of non-pyrethroid insecticides and a new detection method for the gene. Parassitologia 41(1–3):323–326
Feng X, Ruan S, Teng Z, Wang K (2015) Stability and backward bifurcation in a malaria transmission model with applications to the control of malaria in China. Math Biosci 266:52–64
Filipe JA, Riley EM, Drakeley CJ, Sutherland CJ, Ghani AC (2007) Determination of the processes driving the acquisition of immunity to malaria using a mathematical transmission model. PLoS Comput Biol 3:e255
Garba SM, Gumel AB (2010) Effect of cross-immunity on the transmission dynamics of two strains of dengue. Int J Math 87(10):2361–2384
Garba SM, Gumel AB, Abu Bakar MR (2008) Backward bifurcations in dengue transmission dynamics. Math Biosci 215(1):11–25
Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW, Hay SI (2010) Climate change and the global malaria recession. Nature 465:342–345
Gething PW, Patil AP, Smith DL, Guerra CA, Elyazar IR, Johnston GL, Tatem AJ, Ha SI (2011) A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J 10:378
Gething PW, Casey DC, Weiss DJ, Kutz MJ et al (2016) Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. N Engl J Med 375(25):2435–2445
Githeko AK, Brandling-Bennett AD, Beier M, Atieli F, Owaga M, Collins FH (1992) The reservoir of Plasmodium falciparum malaria in a holoendemic area of western Kenya. Trans R Soc Trop Med Hyg 86(4):355–358
Global-Health/Malaria (2019). https://www.gatesfoundation.org/What-We-Do/Global-Health/Malaria. Accessed Jan 2019
Glunt KD, Coetzee M, Huijben S, Koffi AA, Lynch PA, N’Guessan R, Oumbouke WA, Sternberg ED, Thomas MB (2018) Empirical and theoretical investigation into the potential impacts of insecticide resistance on the effectiveness of insecticide-treated bed nets. Evol Appl 11(4):431–441
Hawley WA, Kuile FO, Steketee RS, Nahlen BL et al (2003) Implications of the western Kenya permethrin-treated bed net study for policy, program implementation, and future research. Am J Trop Med Hyg 68(Suppl. 4):168–173
Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, Coetzee M, Simard F, Roch DK, Hinzoumbe CK, Pickett J (2016) Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet 387(10029):1785–8
Himeidan YE, Kweka EJ (2012) Malaria in the East African highlands during the past 30 years: impact of environmental changes. Front Physiol 3(315):1–11
Holzer BR, Egger M, Teuscher T, Koch S, Mboya DM, Smith GD (1993) Childhood anemia in Africa: to transfuse or not transfuse? Acta Trop 55(1–2):47–51
Huijben S, Paaijmans KP (2017) Putting evolution in elimination: winning our ongoing battle with evolving malaria mosquitoes and parasites. Evol Appl 11(4):415–430
Iboi EA, Gumel AB (2018) Mathematical assessment of the roles of temperature and Dengvaxia vaccine on the transmission dynamics of dengue serotypes. Math Biosci 304:25–47
Iboi E, Okuneye K, Sharomi O, Gumel AB (2018) Comments on a mathematical study to control visceral leishmaniasis: an application to South Sudan. Bull Math Biol 80:825–839
Imwong M, Suwannasin K, Kunasol C, Sutawong K, Mayxay M, Rekol H, Dondorp AM (2017) The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong Subregion: a molecular epidemiology observational study. Lancet Infect Dis. https://doi.org/10.1016/S1473-3099(17)30048-8
Jeffery GM, Eyles DE (1954) The duration in the human host of infections with a Panama strain of Plasmodium falciparum. Am J Trop Med Hyg 3:219–224
Johnston GL, Smith DL, Fidock DA (2013) Malaria’s missing number: calculating the human component of \({\cal{R}}_0\) by a within-host mechanistic model of Plasmodium falciparum infection and transmission. PLoS Comput Biol 9(4):e1003025. https://doi.org/10.1371/journal.pcbi.1003025
Kabula B, Kisinza W, Tungu P, Ndege C, Batengana B, Kollo D, Malima R, Kafuko J, Mohamed M, Magesa S (2014) Co-occurrence and distribution of East (L1014S) and West (L1014F) African knock down resistance in Anopheles gambiae sensu lato population of Tanzania. Trop Med Int Health 19(3):331–41
Ketoh GK, Ahadji-Dabla KM, Chabi J, Amoudji AD, Apetogbo GY, Awokou F, Glitho IA (2018) Efficacy of two PBO long lasting insecticidal nets against natural populations of Anopheles gambiae sl in experimental huts, Kolokopé. Togo. PloS One 13(7):e0192492
Killeen GF, Smith TA (2007) Exploring the contributions of bed nets, cattle, insecticides and excitorepellency to malaria control: a deterministic model of mosquito host-seeking behaviour and mortality. Trans R Soc Trop Med Hyg 101(9):867–80
Killeen GF, Chitnis N, Moore SJ, Okumu FO (2011) Target product profile choices for intra-domiciliary malaria vector control pesticide products: repel or kill? Malar J 10(1):207
Kleinschmidt I, Bradley J, Knox T, Mnzava AP, Kafy H et al (2018) Implications of insecticide resistance for malaria vector control with longlasting insecticidal nets: a WHO-coordinated, prospective, international, observational cohort study. Lancet Infect Dis 18(6):640–649
Koffi AA, Alou LPA, Djenontin A, Kabran JPK, Dosso Y, Kone A, Pennetier C (2015) Efficacy of Olyset\(\textregistered \) Duo, a permethrin and pyriproxyfen mixture net against wild pyrethroid-resistant Anopheles gambiae ss from Côte d’Ivoire: an experimental hut trial. Parasite 22:28
Kweka EJ, Lyaruu LJ, Mahande AM (2017) Efficacy of PermaNet\(\textregistered \) 3.0 and PermaNet\(\textregistered \) 2.0 nets against laboratory-reared and wild Anopheles gambiae sensu lato populations in northern Tanzania. Infect Dis Poverty 6(1):11
LaSalle J, Lefschetz S (1976) The stability of dynamical systems. SIAM, Philadelphia
Le Menach A, Takala S, McKenzie FE, Perisse A, Harris A, Flahault A, Smith DL (2007) An elaborated feeding cycle model for reductions in vectorial capacity of night-biting mosquitoes by insecticide-treated nets. Malar J 6(1):10
Levitz L, Janko M, Mwandagalirwa K, Thwai KL, Likwela JL, Tshefu AK, Emch M, Meshnick SR (2018) Effect of individual and community-level bed net usage on malaria prevalence among under-fives in the Democratic Republic of Congo. Malar J 17(1):39
Lines JD, Wilkes TJ, Lyimo EO (1991) Human malaria infectiousness measured by age-specific sporozoite rates in Anopheles gambiae in Tanzania. Parasitology 102:167–177
Macdonald G (1957) The epidemiology and control of malaria. Oxford University Press, Oxford
Malima RC, Magesa SM, Tungu PK, Mwingira V, Magogo FS, Sudi W, Rowland M (2008) An experimental hut evaluation of Olyset\(\textregistered \) nets against anopheline mosquitoes after seven years use in Tanzanian villages. Malar J 7(1):38
Malima R, Tungu PK, Mwingira V, Maxwell C, Magesa SM, Kaur H, Rowland M (2013) Evaluation of the long-lasting insecticidal net interceptor LN: laboratory and experimental hut studies against anopheline and culicine mosquitoes in northeastern Tanzania. Parasites Vectors 6(1):296
Mohammed-Awel J, Numfor E (2017) Optimal insecticide treated bednet coverage and malaria treatment in a malaria-HIV co-infection model. J Biol Dyn 11:160–191
Mohammed-Awel J, Agusto F, Mickens RE, Gumel AB (2018) Mathematical assessment of the role of vector insecticide resistance and feeding/resting behavior on malaria transmission dynamics: optimal control analysis. Infect Dis Model 3:301–321
Mordecai EA, Paaijmans KP, Johnson LR, Balzer C, Ben-Horin T, de Moor E, McNally A, Pawar S, Ryan SJ, Smith TC, Lafferty KD (2013) Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol Lett 16:22–30
Nájera JA, González-Silva M, Alonso PL (2011) Some lessons for the future from the Global Malaria Eradication Programme (1955–1969). PLoS Med 8(1):e1000412
N’Guessan R, Darriet F, Doannio JMC, Chandre F, Carnevale P (2001) Olyset Net\(\textregistered \) efficacy against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus after 3 years field use in Côte d’Ivoire. Med Vet Entomol 15(1):97–104
N’Guessan R, Corbel V, Akogbéto M, Rowland M (2007) Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area. Benin. Emerg Infect Dis 13(2):199–206
N’Guessan R, Asidi A, Boko P, Odjo A, Akogbeto M, Pigeon O, Rowland M (2010) An experimental hut evaluation of PermaNet\(\textregistered \) 3.0, a deltamethrin-piperonyl butoxide combination net, against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes in southern Benin. Trans R Soc Trop Med Hyg 104(12):758–765
Ngufor C, N’Guessan R, Boko P, Odjo A, Vigninou E, Asidi A, Rowland M (2011) Combining indoor residual spraying with chlorfenapyr and long-lasting insecticidal bed nets for improved control of pyrethroid-resistant Anopheles gambiae: an experimental hut trial in Benin. Malar J 10(1):343
Ngufor C, N’Guessan R, Fagbohoun J, Odjo A, Malone D, Akogbeto M, Rowland M (2014) Olyset Duo\(\textregistered \) (a pyriproxyfen and permethrin mixture net): an experimental hut trial against pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus in Southern Benin. PloS One 9(4):e93603
Ngufor C, N’guessan R, Fagbohoun J, Todjinou D, Odjo A, Malone D, Rowland M (2016) Efficacy of the Olyset Duo net against insecticide-resistant mosquito vectors of malaria. Sci Transl Med 8(356):356ra121
Okumu FO, Moore SJ (2011) Combining indoor residual spraying and insecticide-treated bednets for malaria control in Africa: a review of possible outcomes and an outline of suggestions for the future. Malar J 10:208
Okumu FO, Kiware SS, Moore SJ, Killeen GF (2013) Mathematical evaluation of community level impact of combining bed nets and indoor residual spraying upon malaria transmission in areas where the main vectors are Anopheles arabiensis mosquitoes. Parasites Vectors 6(1):17
Okuneye K, Eikenberry SE, Gumel AB (2019) Weather-driven malaria transmission model with gonotrophic and sporogonic cycles. J Biol Dyn 13(1):288–324
Oxborough RM, Kitau J, Matowo J, Feston E, Mndeme R, Mosha FW, Rowland MW (2013) ITN mixtures of chlorfenapyr (pyrrole) and alphacypermethrin (pyrethroid) for control of pyrethroid resistant Anopheles arabiensis and Culex quinquefasciatus. PLoS One 8(2):e55781
Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB (2010) Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci 107:15135–15139
Pennetier C, Bouraima A, Chandre F, Piameu M, Etang J, Rossignol M, Pigeon O (2013) Efficacy of Olyset\(\textregistered \) Plus, a new long-lasting insecticidal net incorporating permethrin and piperonil-butoxide against multi-resistant malaria vectors. PLoS One 8(10):e75134
Protopopoff N, Mosha JF, Lukole E, Charlwood JD, Wright A, Mwalimu CD, Manjurano A, Mosha FW, Kisinza W, Kleinschmidt I, Rowland M (2018) Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet 391(10130):1577–1588
Randriamaherijaona S, Briët OJ, Boyer S, Bouraima A, N’Guessan R, Rogier C, Corbel V (2015) Do holes in long-lasting insecticidal nets compromise their efficacy against pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus? Results from a release-recapture study in experimental huts. Malar J 14(1):332
Reyburn H, Mbatia R, Drakeley C, Bruce J, Carneiro I, Olomi R, Riley EM (2005) Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA 293:1461–1470
Rickman LS, Jones TR, Long GW, Paparello S, Schneider I, Paul CF, Hoffman SL (1990) Plasmodium falciparum-infected Anopheles stephensi inconsistently transmit malaria to humans. Am J Trop Med Hyg 43:441–445
Sama W, Killeen G, Smith T (2004) Estimating the duration of Plasmodium falciparum infection from trials of indoor residual spraying. Am J Trop Med Hyg 70:625–634
Smith DL, Dushoff J, Snow RW, Hay SI (2005) The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 438:492–495
Smith DL, Hay SI, Noor AM, Robert WS (2009) Predicting changing malaria risk after expanded insecticide-treated net coverage in Africa. Trends Parasitol 25(11):511–516. https://doi.org/10.1016/j.pt.2009.08.002
Takken W, Klowden MJ, Chambers GM (1998) Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J Med Entomol 35:639–645
The Alliance for Malaria Prevention (2018) Net mapping Q2 2018. http://allianceformalariaprevention.com/net-mapping-project/. Accessed Jan 2019
Toe KH, Müller P, Badolo A, Traore A, Sagnon N, Dabire RK, Ranson H (2018) Do bednets including piperonyl butoxide offer additional protection against populations of Anopheles gambiae s.l. that are highly resistant to pyrethroids? An experimental hut evaluation in Burkina Faso. Med Vet Entomol 32:407–416
Tungu P, Magesa S, Maxwell C, Malima R, Masue D, Sudi W, Rowland M (2010) Evaluation of PermaNet 3.0 a deltamethrin-PBO combination net against Anopheles gambiae and pyrethroid resistant Culex quinquefasciatus mosquitoes: an experimental hut trial in Tanzania. Malar J 9(1):21
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
Weidong G, Robert JN (2009) Predicting the impact of insecticide-treated bed nets on malaria transmission: the devil is in the detail. Malar J 8:256
Willis DW, Hamon N (2018) Eliminating malaria by 2040 among agricultural households in Africa; potential impact on health, labor productivity, education and gender equality. Gates Open Res 2:33. https://doi.org/10.12688/gatesopenres.12843.2
World Health Organization (2012) World malaria report 2012, Switzerland, Geneva
World Health Organization (2015a) Malaria: draft global technical strategy. Sixty-Eighth World Health Assembly, March 20, 2015
World Health Organization (2015b) World malaria report 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/. Accessed Jan 2019
World Health Organization (2015c) Global technical strategy for malaria 2016–2030. http://www.who.int/malaria/publications/atoz/9789241564991/en/. Accessed Jan 2019
World Health Organization (2015d) Global technical strategy for malaria 2016–2030. World Health Organization, Geneva
World Health Organization (2016) world malaria report 2016. http://apps.who.int/iris/bitstream/10665/252038/1/9789241511711-eng.pdf?ua=1. Accessed Jan 2019
World Health Organization (2017a) Malaria media center: fact sheet, updated April 2017. http://www.who.int/mediacentre/factsheets/fs094/en/
World Health Organization (2017b) Global insecticide resistance database. November 2017. https://www.who.int/malaria/areas/vector_control/en/
World Health Organization (2017c) Achieving and maintaining universal coverage with long-lasting insecticidal nets for malaria control. World Health Organization, Geneva
World Health Organization (2018) World malaria day 2018: ready to beat malaria. https://www.who.int/malaria/media/world-malaria-day-2018/en/. Accessed 10 Mar 2019
World Health Organization (2019) WHO recommended insecticides for indoor residual spraying against malaria vectors. https://www.who.int/neglected_diseases/vector_ecology/vector-control/Insecticides_IRS_22_September_2018.pdf?ua=1. Accessed 6 Apr
Yaro AS, Dao A, Adamou A, Crawford JE, Ribeiro JM, Gwadz R et al (2006) The distribution of hatching time in Anopheles gambiae. Malar J 5:19
Acknowledgements
One of the authors (ABG) is grateful to National Institute for Mathematical and Biological Synthesis (NIMBioS) for funding the Working Group on Climate Change and Vector-borne Diseases (VBDs). NIMBioS is an Institute sponsored by the National Science Foundation, the U.S. Department of Homeland Security, and the U.S. Department of Agriculture through NSF Award #EF-0832858, with additional support from The University of Tennessee, Knoxville. ABG also acknowledges the support, in part, of the Simons Foundation (Award \(\#\)585022). We are very thankful to the two anonymous reviewers and the Handling Editor for their very insightful and constructive comments, which have significantly enhanced the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A: Proof of Lemma 2.1
Proof
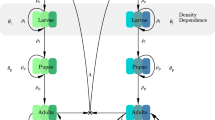
(a) It should be noted, first of all, that the right-hand side of each of the equations of the model (2.1), (2.2),(2.4) is continuous and locally-Lipschitz at \(t=0\). Hence, a solution of the model with non-negative initial conditions exists and is unique in \(\Omega =\Omega _1\times \Omega _2\times \Omega _3\) for all time \(t>0\) (see also Iboi et al. 2018; Okuneye et al. 2019). Furthermore, since \({\left( 1-\frac{E}{K_E} \right) }_+\ge 0,\) it follows from the first equation of the sub-system (2.1) that \(E(t)\le K_E\) for all time \(t>0\). Similarly, it follows from the second equation of the sub-system (2.1) that
so that \(\displaystyle \limsup _{t\rightarrow \infty }L_1(t)\le \frac{\sigma _E K_E}{\sigma _{L_1}+\mu _L}=L^{\diamond }_1.\) Using a similar approach, it can be shown that \(\displaystyle \limsup _{t\rightarrow \infty }L_2(t)\le \frac{\sigma _{L_1} L^{\diamond }_1}{\sigma _{L_2}+\mu _L}=L^{\diamond }_2\), \(\displaystyle \limsup _{t\rightarrow \infty }L_3(t)\le \frac{\sigma _{L_2}L^{\diamond }_2}{\sigma _{L_3}+\mu _L}=L^{\diamond }_3\), \(\displaystyle \limsup _{t\rightarrow \infty }L_4(t)\le \frac{\sigma _{L_3}L^{\diamond }_3}{\sigma _{L_4}+\mu _L}=L^{\diamond }_4\) and \(\displaystyle \limsup _{t\rightarrow \infty }P(t)\le \frac{\sigma _{L_4}L^{\diamond }_4}{\sigma _P +\mu _P}=P^{\diamond }.\) That is, all solutions of the sub-system (2.1) are bounded for all time \(t>0\).
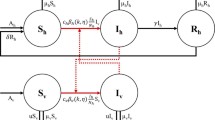
For the boundedness of the solutions of the sub-system (2.2), we consider the following equation (for the rate of change of the total adult mosquito population):
where, \(N_M=A_X+A_Y+A_Z\) (with \(A_X, A_Y, A_Z, Q_1, Q_2, Q_3, R_1\) and \(R_2\) are as defined in Sect. 2). It can be shown that \(Q_2+Q_3-Q_1+R_1+R_2<0\). Hence, Eq. (A-1) can be re-written as
so that,
Hence, the solutions of the equations of the sub-system (2.2) are bounded for all time \(t>0\). Similarly, consider the equation for the rate of change of the total human population, given by:
from which it follows that \(\displaystyle \limsup _{t\rightarrow \infty } N(t)\le \frac{\Pi }{\mu _H}.\) Thus, the solutions of the sub-system (2.4) are bounded for all \(t>0\). Since the solutions of the three sub-systems of the model (2.1), (2.2),(2.4) are bounded, it follows that the solutions of the model are bounded. This concludes the proof of Item (a).
(b) The proof for the invariance of the region \(\Omega _1\) follows from the bounds established in Item (a) (i.e., \(0<\displaystyle \limsup _{t\rightarrow \infty }L_1(t)\le L^{\diamond }_1\), \(0<\displaystyle \limsup _{t\rightarrow \infty }L_j(t)\le L^{\diamond }_j\) and \(0<\displaystyle \limsup _{t\rightarrow \infty }P(t)\le P^{\diamond }\)) and the fact that \({\dot{E}}(t)<0\) whenever \(E(t)>K_E\), \({\dot{L}}_1(t)<0\) whenever \(L_1(t)>L^{\diamond }_1\) and \({\dot{L}}_j(t)<0\) whenever \(L_j(t)>L^{\diamond }_1\) (\(j=2,3,4\)), respectively.
For the invariance of the region \(\Omega _2\), it is convenient to consider the following equation for the rate of change of the total mosquito population given by:
It follows that \({\dot{N}}_M<0\) whenever \(N_M(t)> \frac{f\sigma _P P^{\diamond }}{\mu _M}\). Thus, the region \(\Omega _2\) is invariant with respect to the sub-system (2.2) of the model (2.1), (2.2),(2.4).
Finally, consider the equation for the total human population given by
It follows that \({\dot{N}}_H<0\) whenever \(N_H(t)> \frac{\Pi }{\mu _H}\). Thus, the region \(\Omega _3\) is invariant with respect to the sub-system (2.4) of the model (2.1), (2.2),(2.4). Since the regions \(\Omega _1\), \(\Omega _2\) and \(\Omega _3\) are positively-invariant and attracting, it follows that \(\Omega =\Omega _1\times \Omega _2\times \Omega _3\) is positively-invariant and attracting for the model (2.1), (2.2),(2.4). This concludes the proof of Item (b). \(\square \)
Appendix B: Coefficients of Eq. (3.5)
Appendix C: Equations of the model (2.1), (2.2), (2.4) without bednets intervention
In the absence of the bednet-based intervention, the sub-systems of the model involving the adults and human dynamics, given by Eqs. (2.2) and (2.4), reduce, respectively, to the following sub-systems:
The equations for the aquatic dynamics, given by (2.1), remain unchanged. Hence, the reduced (no-bednets) model consist of the Eqs. (2.1), (C-1), (C-2).
It can be shown, using the next generation operator method (as in Sect. 3), that the basic reproduction number of the reduced model (2.1), (C-1), (C-2) is given by
where,
Substituting the baseline parameter values in Table 4 (for the holo-endemic setting) shows that the worst-case scenario basic reproduction number \((\tilde{{\mathcal {R}}}_0)\) of the model (2.1), (2.2), (2.4), or, equivalently, the reduced model (C-1), (C-2), given by (C-3), is \(\tilde{{\mathcal {R}}}_{0*}\) \(=\) 11.4.
Appendix D: Proof of Theorem 3.2
Proof
The proof of Theorem 3.2 is based on using center manifold theory (Carr 1981; Castillo-Chavez and Song 2004). It is convenient to define the following change of variables for the model (2.1), (2.2), (2.4): \(E=x_1\), \(L_{1}=x_2\), \(L_{2}=x_3\), \(L_{3}=x_4\), \(L_{4}=x_5\), \(P=x_6\), \(S_{X}=x_7\), \(E_{X}=x_8\), \(I_{X}=x_9\), \(S_{Y}=x_{10}\), \(E_{Y}=x_{11}\), \(I_{Y}=x_{12}\), \(S_{Z}=x_{13}\), \(E_{Z}=x_{14}\), \(I_{Z}=x_{15}\), \(S_{Hp}=x_{16}\), \(E_{Hp}=x_{17}\), \(I_{Hp}=x_{18}\), \(R_{Hp}=x_{19}\), \(S_{Hu}=x_{20}\), \(E_{Hu}=x_{21}\), \(I_{Hu}=x_{22}\), \(R_{Hu}=x_{23}\). Using the vector notation \(X=(x_1,\ldots x_{23})^T\) and \(F=(f_1,\ldots f_{23})^T\), the model can then be written in the form \(\frac{dX}{dt} = (f_1,\ldots f_{23})^T\), as follows:
where,
Let \({\mathcal {R}}_0=1\) and suppose, further, that \(\beta _M=\beta _M^*\) is chosen as a bifurcation parameter. Solving for \(\beta _M=\beta _M^*\) from \({\mathcal {R}}_0=1\) gives
The Jacobian of the transformed system (D-1), evaluated at the DFE \(({\mathcal {T}}_2)\) with \(\beta _M=\beta _M^*\), is given by
where,
and,
The Jacobian \(J(\beta _M^*)\) has a simple zero eigenvalue (and all other eigenvalues having negative real parts). Hence, the center manifold theory Carr (1981); Castillo-Chavez and Song (2004) can be used to analyse the dynamics of (D-1) near \(\beta _M=\beta _M^*\). This entails carrying out the following computations.
Eigenvectors of\(J({\mathcal {T}}_2)\mid _{\beta _M=\beta _M^*}\) The Jacobian of the transformed system (D-1), evaluated at the DFE \(({\mathcal {T}}_2)\) with \(\beta _M=\beta _M^*\), has a right and left eigenvectors (associated with the zero eigenvalue) (the expression for the eigenvector \(w_i\) and \(v_i\), \(i=1,2,..,23\), are given in the Supplementary Material).
Computations of bifurcation coefficients of a and b
By computing the associated non-zero partial derivatives of F(x) evaluated the the DFE, it follows from Theorem 4.1 in Castillo-Chavez and Song (2004) that the associated bifurcation coefficients, a and b, are given, respectively, by
and,
Hence, it follows from Theorem 4.1 of Castillo-Chavez and Song (2004) that the transformed model (D-1) undegoes a backward bifurcation at \({\mathcal {R}}_0=1\) if the bifurcation coefficient a (given by (D-2)) is positive. This concludes the proof. \(\square \)
Appendix E: Proof of Theorem 3.3.
Proof
Consider the special case of the model (2.1), (2.2), (2.4) without disease-induced mortality in the host population (i.e., \(\delta _H=0\)). Further, let \({\mathcal {N}}_0>1\) (so that the NDFE, \({\mathcal {T}}_2\), exists) and \(\tilde{{\mathcal {R}}}_0\le 1\). Setting \(\delta _H=0\) in the model (2.1), (2.2), (2.4) gives \(N_H(t)\rightarrow \frac{\Pi }{\mu _H}\) as \(t \rightarrow \infty \), and \({\bar{K}}_{14}=\alpha _H+\mu _H\). Hence, from now on, \(N_H(t)\) is replaced by its limiting value, \(\frac{\Pi }{\mu _H}\). Furthermore, consider the following linear Lyapunov function:
where,
The Lyapunov derivative of \({\mathcal {F}}\) (where a dot represents differentiation with respect to t) is given by:
Since \(S_{Hp}(t)\le N_{Hp}\), \(S_{Hu}(t)\le N_{Hu}\), \(N_{Hp}(t)=\frac{\Pi \,\pi _p}{\mu _H}\) and \(N_{Hu}(t)=\frac{\Pi \,\pi _u}{\mu _H}\) in \(\Omega \) for all \(t>0\), it follows that:
Hence, \({\dot{\mathcal {F}}}\le 0\) if \(\tilde{{\mathcal {R}}}_0<1\) with \({\dot{\mathcal {F}}}= 0\) if and only if \(I_X=I_{Hp}=I_{Hu}=E_Y=0.\) Therefore, \(\mathcal {{F}}\) is a Lyapunov function in \(\Omega \) and it follows from LaSalle’s Invariance Principle (1976) that every solution to the equations in (2.1), (2.2), (2.4) (with \(\delta _H=0\) and initial conditions in \(\Omega \)) converges to \({\mathcal {T}}_2\) as \(t\rightarrow \infty \) That is,
as \(t\rightarrow \infty \) for \(\tilde{{\mathcal {R}}}_0\le 1\). Hence, the NDFE, \({\mathcal {T}}_2\), is globally-asymptotically stable in \(\Omega \) if \(\tilde{{\mathcal {R}}}_0\le 1\) for the special case of the model (2.1), (2.2), (2.4) with \(\delta _H=0\). This completes the proof. \(\square \)
Rights and permissions
About this article
Cite this article
Enahoro, I., Eikenberry, S., Gumel, A.B. et al. Long-lasting insecticidal nets and the quest for malaria eradication: a mathematical modeling approach. J. Math. Biol. 81, 113–158 (2020). https://doi.org/10.1007/s00285-020-01503-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-020-01503-z