Abstract
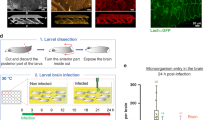
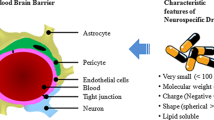
Drosophila melanogaster, a.k.a. the common fruit fly, is a simple organism that may give a rapid, high-throughput response in regard to the therapeutic efficacy of nanoparticles and drugs, while circumventing the high environmental and monetary cost of today’s typical in vivo assays involving more complex animals, along with the immeasurable suffering imposed onto them. Here we give the progress report on our effort to turn D. melanogaster into a model organism for the in vivo testing of Blood-Brain Barrier (BBB) penetration of nano-particles and the treatment of infectious disease. We show that orally ingested superparamagnetic nanoparticles successfully cross the BBB in D. melanogaster and localize to the optic lobes of the third instar larval brain, while causing no adverse effects to the invertebrate organisms. We also show that both orally ingested calcium phosphate nanoparticles and biofilm-forming P. aeruginosa localize to the Drosophila crop, the food storage organ of the fly, which shrinks in response to infection. The model does not induce mortality consequential to infection and the effects of the internalization and proliferation of the microbes are evaluable by measuring the crop parameters, including fluorescence intensity and size. Continued development of these two models could simplify the preclinical testing of medical treatments and of pharmaceutical agents for neurological and infectious disease, while ensuring robust and reliable levels of statistical significance at low cost.
Similar content being viewed by others
References
Ericsson A C, Crim M J, Franklin C L. A brief history of animal modeling. Missouri Medicine, 2013, 110, 201–205.
Jennings B H. Drosophila–A versatile model for biology & medicine. Materials Today, 2011, 14, 190–195.
Wu V M, Schulte J, Hirschi A, Tepass U, Beitel G J. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. Journal of Cell Biology, 2004, 164, 313–323.
Wu V M, Yu M H, Paik R, Banerjee S, Liang Z, Paul S M, Bhat M A, Beitel G J. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development, 2007, 134, 999–1009.
Krench M, Littleton J T. Neurotoxicity pathways in drosophila models of the polyglutamine disorders. Current Topics in Developmental Biology, 2017, 121, 201–223.
Piccolo L L. Drosophila as a model to gain insight into the role of lncRNAs in neurological disorders. Advances in Experimental Medical Biology, 2018, 1076, 119–146.
Bouleau S, Tricoire H. Drosphila models of Alzheimer’s disease: Advances, limits, and perspectives. Journal of Alzheimer’s Disease, 2015, 45, 1015–1038.
Mondotte J A, Saleh M C. Antiviral immune response and the route of infection in Drosophila melanogaster. Advances in Virus Research, 2018, 100, 247–278.
Bergman P, Esfahani S S, Engstrom Y. Drosophila as a model for human diseases–Focus on innate immunity in barrier epithelia. Current Topics in Developmental Biology, 2017, 121, 29–81.
Wong A C N, Vanhove A S, Watnick P I. The interplay between intestinal bacteria and host metabolism in health and disease: Lessons from Drosophila melanogaster. Disease Model Mechanisms, 2016, 9, 271–281.
Singhal N, Jaiswal M. Pathways to neurodegeneration: Lessons learnt from unbiased genetic screens in Drosophila. Journal of Genetics, 2018, 97, 773–781.
Valanne S. Functional genomic analysis of the drosophila immune response. Developmental and Comparative Immunology, 2014, 42, 93–101.
Kislukhin G, Murphy M L, Jafari M, Long A D. Chemotherapy-induced toxicity is highly heritable in Drosophila melanogaster. Pharmacogenetics and Genomics, 2012, 22, 285–289.
Vecchio G. A fruit fly in the nanoworld: Once again Drosophila contributes to environment and human health. Nanotoxicology, 2015, 9, 135–137.
Leeuw T K, Michelle Reith R, Simonette R A, Harden M E, Cherukuri P, Tsyboulski D A, Beckingham K M, Weisman R B. Single-walled carbon nanotubes in the intact organism: Near-IR imaging and biocompatibility studies in Drosophila. Nano Letters, 2007, 7, 2650–2654.
Wu V M, Huynh E, Tang S, Uskoković V. Brain and bone cancer targeting by a ferrofluid composed of superpara-magnetic iron-oxide/silica/carbon nanoparticles (earthicles). Acta Biomaterialia, 2019, 88, 422–447.
Wu V M, Tang S, Uskoković V. Calcium phosphate nano-particles as intrinsic inorganic antimicrobials: The antibacterial effect. ACS Applied Materials and Interfaces, 2018, 10, 34013–34028.
Wu V M, Uskoković V. Waiting for Aπαταω: 250 years later. Foundations of Science, 2019, 24, 617–640.
Uskoković V, Desai T A. Phase composition control of calcium phosphate nanoparticles for tunable drug delivery kinetics and treatment of osteomyelitis. I. Preparation and drug release. Journal of Biomedical Materials Research A, 2013, 101, 1416–1426.
Ghosh S, Wu V M, Pernal S, Uskoković V. Self-setting calcium phosphate cements with tunable antibiotic release rates for advanced bone graft applications. ACS Applied Materials and Interfaces, 2016, 8, 7691–7708.
Uskoković V, Desai T A. Simultaneous bactericidal and osteogenic effect of nanoparticulate calcium phosphate powders loaded with clindamycin on osteoblasts infected with staphylococcus aureus. Materials Science and Engineering C, 2014, 37, 210–222.
Uskoković V, Rau J V. Nonlinear oscillatory dynamics of the hardening of calcium phosphate cements. RSC Advances, 2017, 7, 40517–40532.
Uskoković V, Tang S, Wu V M. On grounds of the memory effect in amorphous and crystalline apatite: Kinetics of crystallization and biological response. ACS Applied Materials and Interfaces, 2018, 10, 14491–14508.
Bhardwaj G, Yazici H, Webster T J. Reducing bacteria and macrophage density on nanophase hydroxyapatite coated onto titanium surfaces without releasing pharmaceutical agents. Nanoscale, 2015, 7, 8416–8427.
Rodrigues B V M, Leite N C S, Cavalcanti B das N, da Silva N S, Marciano F R, Corat E J, Webster T J, Lobo A O. Graphene oxide/multi-walled carbon nanotubes as nano-featured scaffolds for the assisted deposition of nanohydroxyapatite: Characterization and biological evaluation. International Journal of Nanomedicine, 2016, 11, 2569–2585.
Rau J V, Wu V M, Graziani V, Fadeeva I V, Fomin A S, Fosca M, Uskoković V. The bone building blues: Self-hardening copper-doped calcium phosphate cement and its in vitro assessment against mammalian cells and bacteria. Materials Science and Engineering C, 2017, 79, 270–279.
Uskoković V, Iyer M A, Wu V M. One ion to rule them all: Combined antibacterial, osteoinductive and anticancer properties of selenite-incorporated hydroxyapatite. Journal of Materials Chemistry B, 2017, 5, 1430–1445.
Uskoković V, Graziani V, Wu V M, Fadeeva I V, Fomin A S, Presniakov I A, Fosca M, Ortenzi M, Caminiti R, Rau J V. Gold is for the mistress, silver for the maid: Enhanced mechanical properties, osteoinduction and antibacterial activity due to iron doping of tricalcium phosphate bone cements. Materials Science and Engineering C, 2019, 94, 798–810.
Uskoković V, Tang S, Nikolić M G, Marković S, Wu V M. Calcium phosphate nanoparticles as intrinsic inorganic antimicrobials: In search of a key particle property. Biointerphases, 2019, 14, 031001.
Desai T A, Uskoković V. Calcium phosphate nanoparticles: A future therapeutic platform for the treatment of osteomyelitis? Therapeutic Delivery, 2013, 4, 643–645.
Uskoković V, Hoover C, Vukomanović M, Uskoković D P, Desai T A. Osteogenic and antimicrobial nanoparticulate calcium phosphate and/or poly-lactide-co-glycolide powders for the treatment of osteomyelitis. Materials Science and Engineering C, 2013, 33, 3362–3373..
Wu V M, Uskoković V. Calcium phosphate nanoparticles in drosophila melanogaster: The effects of phase composition, crystallinity and the pathway of formation. ACS Biomaterials Science and Engineering, 2017, 3, 2348–2357.
Mulcahy L R, Isabella V M, Lewis K. Pseudomonas aeruginosa biofilms in disease. Microbial Ecology, 2014, 68, 1–12.
Lewis K. Riddle of biofilm resistance. Antimicrobial Agents and Chemotherapy, 2001, 45, 999–1007.
Aliaga L, Mediavilla J D, Cobo F. A clinical index predicting mortality with pseudomonas aeruginosa bacteraemia. Journal of Medical Microbiology, 2002, 51, 615–701.
Hindle S J, Bainton R J. Barrier mechanisms in the Drosophila blood-brain barrier. Frontiers in Neuroscience, 2014, 8, 414.
Unhavaithaya Y, Orr-Weaver T L. Polyploidization of glia in neural development links tissue growth to blood-brain barrier integrity. Genes & Development, 2012, 26, 31–36.
Silies M, Yuva Y, Engelen D, Aho A, Stork T, Klämbt C. Glial cell migration in the eye disc. Journal of Neuroscience, 2007, 27, 13130–13139.
DeSalvo M K, Mayer N, Mayer F, Bainton R J. Physiologic and anatomic characterization of the brain surface glia barrier of Drosophila. Glia, 2011, 59, 1322–1340.
Limmer S, Weiler A, Volkenhoff A, Babatz F, Klambt C. The drosophila blood-brain barrier: Development and function of a glial endothelium. Frontiers in Neuroscience, 2014, 8, 00365.
Skerrett I M, Williams J B. A structural and functional comparison of gap junction channels composed of connexins and innexins. Developmental Neurobiology, 2017, 77, 522–547.
Hindle S J, Munji R N, Dolghih E, Gaskins G, Orng S, Ishimoto H, Soung A, DeSalvo M, Kitamoto T, Keiser M J, Jacobson M P, Daneman R, Bainton R J. Evolutionarily conserved roles for blood-brain barrier xenobiotic transporters in endogenous steroid partitioning and behavior. Cell Reports, 2017, 21, 1304–1316.
Zhang S L, Yue Z F, Arnold D M, Artiushin G, Sehgal A. A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell, 2018, 173, 130–139.
Pernal S, Wu V M, Uskoković V. Hydroxyapatite as a vehicle for the selective effect of superparamagnetic iron oxide nanoparticles against human glioblastoma cells. ACS Applied Materials and Interfaces, 2017, 9, 39283–39302.
Mulcahy H, Sibley C D, Surette M G, Lewenza S. Drosophila melanogaster as an animal model for the study of Pseudomonas aeruginosa biofilm infections in vivo. PLoS Pathogens, 2011, 7, e1002299.
Uskoković V. Entering the era of nanoscience: Time to be so small. Journal of Biomedical Nanotechnology, 2013, 9, 1441–1470.
Zheng Z H, Lauritzen J S, Perlman E, Robinson C G, Nichols M, Milkie D, Torrens O, Price J, Fisher C B, Sharifi N, Calle-Schuler S A, Kmecova L, Ali I J, Karsh B, Trautman E T, Bogovic J A, Hanslovsky P, Jefferis G S X E, Kazhdan M, Khairy K, Saalfeld S, Fetter R D, Bock D D. A complete electron microscopy volume of the brain of adult drosophila melanogaster. Cell, 2018, 174, 730–743.
Cardona A, Saalfeld S, Tomancak P, Hartenstein V. Drosophila brain development: Closing the gap between a macroarchitectural and microarchitectural approach. Cold Spring Harbor Symposia on Quantitative Biology, 2009, 74, 235–248.
Wong R, Piper M D W, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS One, 2009, 4, e6063.
Shell B C, Schmitt R E, Lee K M, Johnson J C, Chung B Y, Pletcher S D, Grotewiel M. Measurement of solid food intake in Drosophila via consumption-excretion of a dye tracer. Scientific Reports, 2018, 8, 11536.
D’Argenio D A, Gallagher L A, Berg C A, Manoil C. Drosophila as a model host for pseudomonas aeruginosa infection. Journal of Bacteriology, 2001, 183, 1466–1471.
Chugani S A, Whiteley M, Lee K M, D’Argenio D, Manoil C, Greenberg E P. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences of the USA, 2001, 98, 2752–2757.
Siva-Jothy J A, Prakash A, Vasanthakrishnan R B, Monteith K M, Vale P F. Oral bacterial infection and shedding in drosophila melanogaster. Journal of Visual Experiments, 2018, 135, e57676.
Donabedian H, Andriole V T. Synergy of vancomycin with penicillins and cephalosporins against pseudomonas, klebsiella, and serratia. Yale Journal of Biological Medicine, 1977, 50, 165–176.
Ghosh S, Patil S, Ahire M, Kitture R, Kale S, Pardesi K, Cameotra S S, Bellare J, Dhavale D D, Jabgunde A, Chopade B A. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. International Journal of Nanomedicine, 2012, 7, 483–496.
Uskoković V, Ghosh S, Wu V M. Antimicrobial hydroxyapatite-gelatin-silica composite pastes with tunable setting properties. Journal of Materials Chemistry B, 2017, 5, 6065–6080.
Wu V M, Mickens J, Uskoković V. Bisphosphonate-functionalized calcium phosphate nanoparticles for the delivery of the bromodomain inhibitor JQ1 in the treatment of osteosarcoma. ACS Applied Materials and Interfaces, 2017, 9, 25887–25904.
Uskoković V, Desai T A. Phase composition control of calcium phosphate nanoparticles for tunable drug delivery kinetics and treatment of osteomyelitis. II. Antibacterial and osteoblastic response. Journal of Biomedical Materials Research A, 2013, 101, 1427–1436.
Sarkar S, Heise M T. Mouse models as resources for studying infectious diseases. Clinical Therapeutics, 2019, 41, 1912–1922.
Sohet F, Daneman R. Genetic mouse models to study blood-brain barrier development and function. Fluids and Barriers of the CNS, 2013, 10, 3.
Colby L A, Quenee L E, Zitzow L A. Considerations for infectious disease research studies using animals. Comparative Medicine, 2017, 67, 222–231.
Schickore J. Through thousands of errors we reach the truth–But how? On the epistemic roles of error in scientific practice. Studies in History and Philosophy of Modern Physics, 2005, 36, 539–556.
Uskoković V. On the light doves and learning on mistakes. Axiomathes: An International Journal in Ontology and Cognitive Systems, 2009, 19, 17–50.
Montale E. Posthumous Diary. Turtle Point Press, New York, USA, 2001.
Acknowledgment
The authors thank Shreya Ghosh from the Uskoković Lab at University of Illinois and Sean Tang from the Uskoković Lab at Chapman University for assistance with the fruit fly analyses and acknowledge the intramural University of Illinois at Chicago funds and the National Institute of Health grant R00-DE021416 for support. There are no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, V.MT., Uskoković, V. Fruit Fly as a Model Organism for Blood-Brain Barrier Penetration and Infectious Disease in the Nanomedical Niche. J Bionic Eng 17, 553–569 (2020). https://doi.org/10.1007/s42235-020-0044-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-020-0044-1