Abstract
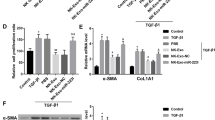
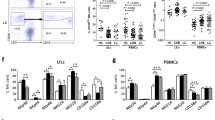
Activation of hepatic stellate cells (HSCs) is a prominent driver of liver fibrosis. This study was designed to investigate the effect of exosomes derived from natural killer (NK) cells on HSC activation and liver fibrosis. The exosomes were isolated from NK-92MI cells (NK-Exo) and identified by transmission electron microscopy, nanoparticle tracking analysis, and western blot. Then NK-Exo was administered into TGF-β1-treated LX-2 cells (human HSC line) and mice with CCl4-induced liver fibrosis. LX-2 cell proliferation was determined by CCK-8 assay. The levels of α-SMA and CoL1A1 were measured by qRT-PCR and western blot to evaluate HSC activation. Serum levels of AST and ALT were measured. Hematoxylin–eosin, Masson staining, and Sirius Red staining were performed to assess the pathological changes and collagen deposition. Cell supernatant derived from NK-92MI cells inhibited TGF-β1-induced HSC proliferation and activation in LX-2 cells, and this effect was counteracted by the exosome inhibitor GW4869. Further assays confirmed that NK-Exo treatment significantly inhibited TGF-β1-induced HSC proliferation and activation. Moreover, NK-Exo administration alleviated CCl4-induced liver fibrosis in mice. NK-Exo inhibited TGF-β1-induced HSC activation and CCl4-induced liver fibrosis.




Similar content being viewed by others
References
Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55. https://doi.org/10.1016/j.mam.2018.09.002.
Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–422. https://doi.org/10.1016/j.addr.2017.05.007.
Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, et al. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118(10):2784–92. https://doi.org/10.1182/blood-2011-04-347070.
Fasbender F, Widera A, Hengstler JG, Watzl C. Natural killer cells and liver fibrosis. Front Immunol. 2016;7:19. https://doi.org/10.3389/fimmu.2016.00019.
Gur C, Doron S, Kfir-Erenfeld S, Horwitz E, Abu-Tair L, Safadi R, et al. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut. 2012;61(6):885–93. https://doi.org/10.1136/gutjnl-2011-301400.
Glassner A, Eisenhardt M, Kramer B, Korner C, Coenen M, Sauerbruch T, et al. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab Investig. 2012;92(7):967–77. https://doi.org/10.1038/labinvest.2012.54.
Zimmermann HW, Mueller JR, Seidler S, Luedde T, Trautwein C, Tacke F. Frequency and phenotype of human circulating and intrahepatic natural killer cell subsets is differentially regulated according to stage of chronic liver disease. Digestion. 2013;88(1):1–16. https://doi.org/10.1159/000350821.
Jeong WI, Park O, Suh YG, Byun JS, Park SY, Choi E, et al. Suppression of innate immunity (natural killer cell/interferon-gamma) in the advanced stages of liver fibrosis in mice. Hepatology (Baltimore, MD). 2011;53(4):1342–51. https://doi.org/10.1002/hep.24190.
Jiao J, Ooka K, Fey H, Fiel MI, Rahmman AH, Kojima K, et al. Interleukin-15 receptor alpha on hepatic stellate cells regulates hepatic fibrogenesis in mice. J Hepatol. 2016;65(2):344–53. https://doi.org/10.1016/j.jhep.2016.04.020.
Chen L, Yao X, Yao H, Ji Q, Ding G, Liu X. Exosomal miR-103-3p from LPS-activated THP-1 macrophage contributes to the activation of hepatic stellate cells. FASEB J. 2020. https://doi.org/10.1096/fj.201902307RRR.
Shoae-Hassani A, Hamidieh AA, Behfar M, Mohseni R, Mortazavi-Tabatabaei SA, Asgharzadeh S. NK cell-derived exosomes from NK cells previously exposed to neuroblastoma cells augment the antitumor activity of cytokine-activated NK cells. J Immunother. 2017;40(7):265–76. https://doi.org/10.1097/cji.0000000000000179.
Neviani P, Wise PM, Murtadha M, Liu CW, Wu CH, Jong AY, et al. Natural killer-derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res. 2019;79(6):1151–64. https://doi.org/10.1158/0008-5472.can-18-0779.
Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH, et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7(10):2732–45. https://doi.org/10.7150/thno.18752.
Yang JX, Xie P, Li YS, Wen T, Yang XC. Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell Signal. 2019. https://doi.org/10.1016/j.cellsig.2019.109504.
Huang W, Huang F, Lei Z, Luo H. LncRNA SNHG11 promotes proliferation, migration, apoptosis, and autophagy by regulating hsa-miR-184/AGO2 in HCC. Onco Targets Ther. 2020;13:413–21. https://doi.org/10.2147/OTT.S237161.
Kojima Y, Tsuchiya A, Ogawa M, Nojiri S, Takeuchi S, Watanabe T, et al. Mesenchymal stem cells cultured under hypoxic conditions had a greater therapeutic effect on mice with liver cirrhosis compared to those cultured under normal oxygen conditions. Regen Ther. 2019;11:269–81. https://doi.org/10.1016/j.reth.2019.08.005.
Wang M, Yang ZK, Liu H, Li RQ, Liu Y, Zhong WJ. Genipin inhibits the scleral expression of miR-29 and MMP2 and promotes COL1A1 expression in myopic eyes of guinea pigs. Graefe's Arch Clin Exp Ophthalmol. 2020. https://doi.org/10.1007/s00417-020-04634-7.
Zheng J, Yu F, Dong P, Wu L, Zhang Y, Hu Y, et al. Long non-coding RNA PVT1 activates hepatic stellate cells through competitively binding microRNA-152. Oncotarget. 2016;7(39):62886–97. https://doi.org/10.18632/oncotarget.11709.
Yang JJ, Yang Y, Zhang C, Li J, Yang Y. Epigenetic silencing of LncRNA ANRIL enhances liver fibrosis and HSC activation through activating AMPK pathway. J Cell Mol Med. 2020;24(4):2677–87. https://doi.org/10.1111/jcmm.14987.
Prestigiacomo V, Weston A, Suter-Dick L. Rat multicellular 3D liver microtissues to explore TGF-beta1 induced effects. J Pharmacol Toxicol Methods. 2020;101:106650. https://doi.org/10.1016/j.vascn.2019.106650.
Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130(2):435–52. https://doi.org/10.1053/j.gastro.2005.10.055.
Gao B, Radaeva S, Jeong WI. Activation of natural killer cells inhibits liver fibrosis: a novel strategy to treat liver fibrosis. Expert Rev Gastroenterol Hepatol. 2007;1(1):173–80. https://doi.org/10.1586/17474124.1.1.173.
Shen M, Ren X. New insights into the biological impacts of immune cell-derived exosomes within the tumor environment. Cancer Lett. 2018;431:115–22. https://doi.org/10.1016/j.canlet.2018.05.040.
Fu J, Du H, Zhang X, Xu X. Pharmacological Inhibition of Transient Receptor Potential Vanilloid 4 (TRPV4) channel alleviates carbon tetrachloride-induced liver fibrosis in mice. J Nippon Med Sch. 2019;86(5):258–62. https://doi.org/10.1272/jnms.JNMS.2019_86-407.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
All animal experiments were conducted under university guidelines and approved by the ethical committee for Animal Care and Use of the Xiangya Hospital of Central South University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Wang, Y. & Quan, J. Exosomes derived from natural killer cells inhibit hepatic stellate cell activation and liver fibrosis. Human Cell 33, 582–589 (2020). https://doi.org/10.1007/s13577-020-00371-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00371-5