Abstract
Purpose
Fedratinib is an oral, selective Janus kinase 2 inhibitor that is approved in the United States for the treatment of patients with intermediate-2 or high-risk myelofibrosis. Pharmacokinetics and tolerability of fedratinib in subjects with renal impairment (RI) and hepatic impairment (HI) were evaluated in two separate studies.
Methods
In the renal study, male and female subjects with stable, chronic mild, moderate, and severe RI, as well as those with end-stage renal disease, were included. The hepatic study included subjects with stable, chronic mild HI. Both were phase 1, multicenter, open-label, single-dose studies, and included matched healthy subjects. Subjects received a single oral dose of fedratinib 300 mg on day 1, were discharged on day 4, returned for clinical visits on days 5–12, and had their end-of-study visit between days 14 and 16.
Results
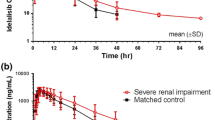
Thirty-six and 17 subjects were included in the renal and hepatic studies, respectively. In the renal study, fedratinib area under the plasma concentration–time curve from time 0 to infinity (AUCinf) was 1.9- and 1.5-fold higher in subjects with severe and moderate RI, respectively, than in matched healthy subjects. In the hepatic study, fedratinib AUCinf did not appreciably differ between subjects with mild HI and matched healthy subjects. Overall, most treatment–emergent adverse events were gastrointestinal and mild.
Conclusion
Mild RI and HI do not necessitate fedratinib dosage adjustments. Subjects with moderate RI should be monitored (with dosage adjustments made as necessary), whereas those with severe RI should receive a daily dose of 200 mg, reduced from the indicated dose of 400 mg.

Similar content being viewed by others
References
Celgene Corporation (2019) Inrebic (fedratinib) [package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf. Accessed 20 May 2020
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, Silverman MH, Gilliland DG, Shorr J, Tefferi A (2011) Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 29(7):789–796. https://doi.org/10.1200/JCO.2010.32.8021
Pardanani A, Tefferi A, Jamieson C, Gabrail NY, Lebedinsky C, Gao G, Liu F, Xu C, Cao H, Talpaz M (2015) A phase 2 randomized dose-ranging study of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J 5:e335. https://doi.org/10.1038/bcj.2015.63
Zhang M, Xu CR, Shamiyeh E, Liu F, Yin JY, von Moltke LL, Smith WB (2014) A randomized, placebo-controlled study of the pharmacokinetics, pharmacodynamics, and tolerability of the oral JAK2 inhibitor fedratinib (SAR302503) in healthy volunteers. J Clin Pharmacol 54(4):415–421. https://doi.org/10.1002/jcph.218
Zhang M, Xu C, Ma L, Shamiyeh E, Yin J, von Moltke LL, Smith WB (2015) Effect of food on the bioavailability and tolerability of the JAK2-selective inhibitor fedratinib (SAR302503): results from two phase I studies in healthy volunteers. Clin Pharmacol Drug Dev 4(4):315–321. https://doi.org/10.1002/cpdd.161
Keller F, Maiga M, Neumayer HH, Lode H, Distler A (1984) Pharmacokinetic effects of altered plasma protein binding of drugs in renal disease. Eur J Drug Metab Pharmacokinet 9(3):275–282. https://doi.org/10.1007/BF03189651
Verbeeck RK, Horsmans Y (1998) Effect of hepatic insufficiency on pharmacokinetics and drug dosing. Pharm World Sci 20(5):183–192. https://doi.org/10.1023/a:1008656930082
Verbeeck RK (2008) Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol 64(12):1147–1161. https://doi.org/10.1007/s00228-008-0553-z
Verbeeck RK, Musuamba FT (2009) Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol 65(8):757–773. https://doi.org/10.1007/s00228-009-0678-8
Abu-Hilal M, Tawaker J (2009) Portal hypertension secondary to myelofibrosis with myeloid metaplasia: a study of 13 cases. World J Gastroenterol 15(25):3128–3133. https://doi.org/10.3748/wjg.15.3128
Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, Vannucchi AM, Mesa RA, Demory JL, Barosi G, Rumi E, Tefferi A (2009) New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 113(13):2895–2901. https://doi.org/10.1182/blood-2008-07-170449
Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, Guglielmelli P, Pungolino E, Caramella M, Maffioli M, Pascutto C, Lazzarino M, Cazzola M, Tefferi A (2010) A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 115(9):1703–1708. https://doi.org/10.1182/blood-2009-09-245837
Tremblay D, Putra J, Vogel A, Winters A, Hoffman R, Schiano TD, Fiel MI, Mascarenhas JO (2019) The implications of liver biopsy results in patients with myeloproliferative neoplasms being treated with ruxolitinib. Case Rep Hematol 2019:3294046. https://doi.org/10.1155/2019/3294046
Said SM, Leung N, Sethi S, Cornell LD, Fidler ME, Grande JP, Herrmann S, Tefferi A, D'Agati VD, Nasr SH (2011) Myeloproliferative neoplasms cause glomerulopathy. Kidney Int 80(7):753–759. https://doi.org/10.1038/ki.2011.147
Christensen AS, Moller JB, Hasselbalch HC (2014) Chronic kidney disease in patients with the Philadelphia-negative chronic myeloproliferative neoplasms. Leuk Res 38(4):490–495. https://doi.org/10.1016/j.leukres.2014.01.014
Baek SW, Moon JY, Ryu H, Choi YS, Song IC, Lee HJ, Yun HJ, Kim S, Jo DY (2018) Chronic kidney disease in the BCR-ABL1-negative myeloproliferative neoplasm: a single-center retrospective study. Korean J Intern Med 33(4):790–797. https://doi.org/10.3904/kjim.2016.263
Fukuda Y, Araki M, Yamamoto K, Morishita S, Inano T, Misawa K, Ochiai T, Edahiro Y, Imai M, Yasuda H, Gotoh A, Ohsaka A, Komatsu N (2019) Evidence for prevention of renal dysfunction associated with primary myelofibrosis by cytoreductive therapy. Haematologica 104(11):e506–e509. https://doi.org/10.3324/haematol.2018.208876
Strati P, Abdelrahim M, Selamet U, Page VD, Pierce SA, Verstovsek S, Abudayyeh A (2019) Ruxolitinib therapy is associated with improved renal function in patients with primary myelofibrosis. Ann Hematol 98(7):1611–1616. https://doi.org/10.1007/s00277-019-03708-9
US Food and Drug Administration (2003) Guidance for industry: pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacokinetics-patients-impaired-hepatic-function-study-design-data-analysis-and-impact-dosing-and. Accessed 18 Nov 2019
US Food and Drug Administration (2010) Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacokinetics-patients-impaired-renal-function-study-design-data-analysis-and-impact-dosing-and. Accessed 18 Nov 2019
Ogasawara K, Zhou S, Krishna G, Palmisano M, Li Y (2019) Population pharmacokinetics of fedratinib in patients with myelofibrosis, polycythemia vera, and essential thrombocythemia. Cancer Chemother Pharmacol 84(4):891–898. https://doi.org/10.1007/s00280-019-03929-9
Docci D, Bilancioni R, Pistocchi E, Mosconi G, Turci F, Salvi G, Baldrati L, Orsi C (1985) Serum alpha-1-acid glycoprotein in chronic renal failure. Nephron 39(3):160–163. https://doi.org/10.1159/000183364
Koiso K, Akaza H, Kikuchi K, Aoyagi K, Ohba S, Miyazaki M, Ito M, Sueyoshi T, Matsushima H, Kamimura H, Watanabe T, Higuchi S (1996) Pharmacokinetics of tamsulosin hydrochloride in patients with renal impairment: effects of alpha 1-acid glycoprotein. J Clin Pharmacol 36(11):1029–1038. https://doi.org/10.1177/009127009603601107
Wolzt M, Fabrizii V, Dorner GT, Zanaschka G, Leufkens P, Krauwinkel WJ, Eichler HG (1998) Pharmacokinetics of tamsulosin in subjects with normal and varying degrees of impaired renal function: an open-label single-dose and multiple-dose study. Eur J Clin Pharmacol 54(4):367–373. https://doi.org/10.1007/s002280050477
Matsushima H, Kamimura H, Soeishi Y, Watanabe T, Higuchi S, Miyazaki M (1999) Plasma protein binding of tamsulosin hydrochloride in renal disease: role of alpha1-acid glycoprotein and possibility of binding interactions. Eur J Clin Pharmacol 55(6):437–443. https://doi.org/10.1007/s002280050653
Funding
The clinical trial reported in this manuscript was sponsored by Sanofi. Medical writing support was provided by Shawn Vahabzadeh, PharmD, of MediTech Media, Ltd, and funded by Bristol Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KO, MP, and GK are employees of and hold equity ownership in Bristol Myers Squibb. WBS declares no conflict of interest. CX are employed by and hold equity ownership in Sanofi and JY is an employee of Sanofi.
Research involving human participants and/or animals
All study procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ogasawara, K., Smith, W.B., Xu, C. et al. Pharmacokinetics and tolerability of fedratinib, an oral, selective Janus kinase 2 inhibitor, in subjects with renal or hepatic impairment. Cancer Chemother Pharmacol 85, 1109–1117 (2020). https://doi.org/10.1007/s00280-020-04084-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04084-2