Abstract
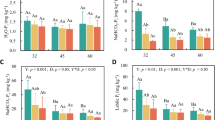
Because climate change is predicted to have a strong impact on high-altitude ecosystems, a better knowledge of litter decomposition in alpine ecosystems is critical to improve our predictions of the effect of climate change on ecosystem processes and services such as nutrient cycling, carbon sequestration, and below-ground biodiversity. To evaluate the effects of vegetation types [alpine shrubland (AS) and alpine meadow (AM)] and litter quality on litter decomposition and related biochemical processes, the decomposition of leaf litter of two dominant shrub species, Sorbus rufopilosa (SR, high quality) and Rhododendron lapponicum (RL, low quality), was studied using the litterbag method in an alpine treeline ecotone on the eastern Tibetan Plateau. After 1 year of decomposition, cellulolytic enzyme activities and gram-negative bacterial biomass were higher in shrubland than in meadow. However, higher fungal biomass, fungal/bacteria ratio and ligninolytic activity were observed in meadow than in shrubland after 2 years of decomposition. During the first year of decomposition, litter decomposition was faster in shrubland than in meadow probably due to the home-field advantage (HFA) effect and the bacteria-dominated decomposition, whereas in later decomposition stages, litter decomposition was faster in meadow than in shrubland, as the HFA effect diminished and fungal-dominated decomposition of recalcitrant components took over. These results indicated that litter quality effects were generally strongest in the first year and diminished in later stages when the effect of vegetation type in incubation sites developed.





Similar content being viewed by others
References
Aerts R. 2006. The freezer defrosting: global warming and litter decomposition rates in cold biomes. Journal of Ecology 94(4):713–24.
Aerts R, Callaghan TV, Dorrepaal E, Van Logtestijn RSP, Cornelissen JHC. 2012. Seasonal climate manipulations have only minor effects on litter decomposition rates and N dynamics but strong effects on litter P dynamics of sub-arctic bog species. Oecologia 170(3):809–19.
Anderson MJ. 2001. Permutation tests for univariate or multivariate analysis of variance and regression. Canadian Journal of Fisheries and Aquatic Sciences 58(3):626–39.
Arora DS, Chander M, Gill PK. 2002. Involvement of lignin peroxidase, manganese peroxidase and laccase in degradation and selective ligninolysis of wheat straw. International Biodeterioration & Biodegradation 50(2):115–20.
Austin AT, Vivanco L, González-Arzac A, Pérez LI. 2014. There’s no place like home? An exploration of the mechanisms behind plant litter–decomposer affinity in terrestrial ecosystems. New Phytologist 204(2):307–14.
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moore JC, Wall DH. 2009. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biology and Biochemistry 41(3):606–10.
Berg B. 2000. Litter decomposition and organic matter turnover in northern forest soils. Forest Ecology and Management 133(1–2):13–22.
Berg B, Mcclaugherty C. 1989. Nitrogen and Phosphorus Release from decomposing litter in relation to the disappearance of lignin. Canadian Journal of Botany 67(4):1148–56.
Berg B, McClaugherty C. 2014. Plant litter: decomposition, humus formation, carbon sequestration. Berlin: Springer.
Berger TW, Duboc O, Djukic I, Tatzber M, Gerzabek MH, Zehetner F. 2015. Decomposition of beech (Fagus sylvatica) and pine (Pinus nigra) litter along an Alpine elevation gradient: decay and nutrient release. Geoderma 251:92–104.
Bossio DA, Scow KM. 1998. Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microbial Ecology 35(3):265–78.
Bottino F, Cunha-Santino MB, Bianchini I Jr. 2016. Cellulase activity and dissolved organic carbon release from lignocellulose macrophyte-derived in four trophic conditions. Brazilian Journal of Microbiology 47(2):352–8.
Bradford MA, Berg B, Maynard DS, Wieder WR, Wood SA, Cornwell W. 2016. Understanding the dominant controls on litter decomposition. Journal of Ecology 104(1):229–38.
Bray JR, Curtis JT. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs 27(4):325–49.
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E. 2013. The Microbial Efficiency Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Global Change Biology 19(4):988–95.
Criquet S. 2002. Measurement and characterization of cellulase activity in sclerophyllous forest litter. Journal of Microbiological Methods 50(2):165–73.
Criquet S, Tagger S, Vogt G, Iacazio G, Le Petit J. 1999. Laccase activity of forest litter. Soil Biology and Biochemistry 31(9):1239–44.
Dilly O, Bloem J, Vos A, Munch JC. 2004. Bacterial diversity in agricultural soils during litter decomposition. Appl Environ Microbiol 70(1):468–74.
Djukic I, Zehetner F, Mentler A, Gerzabek MH. 2010. Microbial community composition and activity in different Alpine vegetation zones. Soil Biology and Biochemistry 42(2):155–61.
Fanin N, Kardol P, Farrell M, Nilsson M-C, Gundale MJ, Wardle DA. 2019. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biology and Biochemistry 128:111–14.
Frostegård Å, Tunlid A, Bååth E. 2011. Use and misuse of PLFA measurements in soils. Soil Biology and Biochemistry 43(8):1621–5.
Frouz J. 2018. Effects of soil macro-and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 332:161–72.
Gavazov KS. 2010. Dynamics of alpine plant litter decomposition in a changing climate. Plant and Soil 337(1–2):19–32.
Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH, Hättenschwiler S. 2010. Diversity meets decomposition. Trends in Ecology & Evolution 25(6):372–80.
Groffman PM, Driscoll CT, Fahey TJ, Hardy JP, Fitzhugh RD, Tierney GL. 2001. Effects of mild winter freezing on soil nitrogen and carbon dynamics in a northern hardwood forest. Biogeochemistry 56(2):191–213.
Harsch MA, Hulme PE, McGlone MS, Duncan RP. 2009. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecology Letters 12(10):1040–9.
Hobbie SE. 1996. Temperature and plant species control over litter decomposition in Alaskan tundra. Ecological Monographs 66(4):503–22.
Horn SJ, Vaaje-Kolstad G, Westereng B, Eijsink VG. 2012. Novel enzymes for the degradation of cellulose. Biotechnol Biofuels 5(1):45.
Kaiser C, Koranda M, Kitzler B, Fuchslueger L, Schnecker J, Schweiger P, Rasche F, Zechmeister-Boltenstern S, Sessitsch A, Richter A. 2010. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytologist 187(3):843–58.
Kara O, Bolat I, Cakıroglu K, Senturk M. 2014. Litter decomposition and microbial biomass in temperate forests in Northwestern Turkey. Journal of Soil Science and Plant Nutrition 14(1):31–41.
Klotzbücher T, Kaiser K, Guggenberger G, Gatzek C, Kalbitz K. 2011. A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 92(5):1052–62.
Konestabo HS, Michelsen A, Holmstrup M. 2007. Responses of springtail and mite populations to prolonged periods of soil freeze-thaw cycles in a sub-arctic ecosystem. Applied Soil Ecology 36(2–3):136–46.
Körner C. 2012. Alpine treelines: functional ecology of the global high elevation tree limits. Basel: Springer.
Kourtev PS, Ehrenfeld JG, Haggblom M. 2003. Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biology and Biochemistry 35(7):895–905.
Kramer C, Gleixner G. 2008. Soil organic matter in soil depth profiles: distinct carbon preferences of microbial groups during carbon transformation. Soil Biology and Biochemistry 40(2):425–33.
Krishna MP, Mohan M. 2017. Litter decomposition in forest ecosystems: a review. Energy, Ecology and Environment 2(4):236–49.
Lazzaro A, Hilfiker D, Zeyer J. 2015. Structures of microbial communities in alpine soils: seasonal and elevational effects. Frontiers in Microbiology 6:1330.
LeBauer DS. 2010. Litter degradation rate and β-glucosidase activity increase with fungal diversity. Canadian Journal of Forest Research 40(6):1076–85.
Liang E, Wang Y, Piao S, Lu X, Camarero JJ, Zhu H, Zhu L, Ellison AM, Ciais P, Peñuelas J. 2016. Species interactions slow warming-induced upward shifts of treelines on the Tibetan Plateau. Proceedings of the National Academy of Sciences of the United States of America 113(16):4380–5.
Liu Y, Chen Y, Zhang J, Yang W, Peng Z, He X, Deng C, He R. 2016. Changes in foliar litter decomposition of woody plants with elevation across an alpine forest–tundra ecotone in eastern Tibet Plateau. Plant Ecology 217(5):495–504.
Löffler UCM, Cypionka H, Löffler J. 2008. Soil microbial activity along an arctic-alpine altitudinal gradient from a seasonal perspective. European Journal of Soil Science 59(5):842–54.
Margesin R, Jud M, Tscherko D, Schinner F. 2009. Microbial communities and activities in alpine and subalpine soils. FEMS Microbiology Ecology 67(2):208–18.
Moore TR, Trofymow JA, Prescott CE, Fyles J, Titus BD. 2006. Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests. Ecosystems 9(1):46–62.
Mooshammer M, Hofhansl F, Frank AH, Wanek W, Hammerle I, Leitner S, Schnecker J, Wild B, Watzka M, Keiblinger KM, Zechmeister-Boltenstern S, Richter A. 2017. Decoupling of microbial carbon, nitrogen, and phosphorus cycling in response to extreme temperature events. Science Advances 3(5):e1602781.
Osono T, Takeda H. 2004. Accumulation and release of nitrogen and phosphorus in relation to lignin decomposition in leaf litter of 14 tree species. Ecological Research 19(6):593–602.
Osono T, Takeda H. 2006. Fungal decomposition of Abies needle and Betula leaf litter. Mycologia 98(2):172–9.
Pérez-Harguindeguy N, Díaz S, Cornelissen JHC, Vendramini F, Cabido M, Castellanos A. 2000. Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant and Soil 218(1–2):21–30.
Prescott CE. 2010. Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101(1–3):133–49.
Schimel JP, Clein JS. 1996. Microbial response to freeze-thaw cycles in tundra and taiga soils. Soil Biology and Biochemistry 28(8):1061–6.
Schinner F. 1982. Soil Microbial Activities and Litter Decomposition Related to Altitude. Plant and Soil 65(1):87–94.
Schneider T, Keiblinger KM, Schmid E, Sterflinger-Gleixner K, Ellersdorfer G, Roschitzki B, Richter A, Eberl L, Zechmeister-Boltenstern S, Riedel K. 2012. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J 6(9):1749–62.
Sinsabaugh RL, Linkins AE. 1993. Statistical Modeling of Litter Decomposition from Integrated Cellulase Activity. Ecology 74(5):1594–7.
Sjögersten S, Wookey PA. 2004. Decomposition of mountain birch leaf litter at the forest-tundra ecotone in the Fennoscandian mountains in relation to climate and soil conditions. Plant and Soil 262(1–2):215–27.
Steinwandter M, Schlick-Steiner BC, Steiner FM, Seeber J. 2019. One plus one is greater than two: mixing litter types accelerates decomposition of low-quality alpine dwarf shrub litter. Plant and Soil 438(1–2):405–19.
Tlaskal V, Voriskova J, Baldrian P. 2016. Bacterial succession on decomposing leaf litter exhibits a specific occurrence pattern of cellulolytic taxa and potential decomposers of fungal mycelia. FEMS Microbiology Ecology 92(11):fiw177.
Valaskova V, Snajdr J, Bittner B, Cajthaml T, Merhautova V, Hoffichter M, Baldrian P. 2007. Production of lignocellulose-degrading enzymes and degradation of leaf litter by saprotrophic basidiomycetes isolated from a Quercus petraea forest. Soil Biology and Biochemistry 39(10):2651–60.
Vanderbilt KL, White CS, Hopkins O, Craig JA. 2008. Aboveground decomposition in arid environments: Results of a long-term study in central New Mexico. Journal of Arid Environments 72(5):696–709.
Vauramo S, Setälä H. 2011. Decomposition of labile and recalcitrant litter types under different plant communities in urban soils. Urban Ecosystems 14(1):59–70.
Veen GF, Freschet GT, Ordonez A, Wardle DA. 2015a. Litter quality and environmental controls of home-field advantage effects on litter decomposition. Oikos 124(2):187–95.
Veen GF, Keiser AD, van der Putten WH, Wardle DA. 2018. Variation in home-field advantage and ability in leaf litter decomposition across successional gradients. Functional Ecology 32(6):1563–74.
Veen GF, Sundqvist MK, Wardle DA. 2015b. Environmental factors and traits that drive plant litter decomposition do not determine home-field advantage effects. Functional Ecology 29(7):981–91.
Vesterdal L. 1999. Influence of soil type on mass loss and nutrient release from decomposing foliage litter of beech and Norway spruce. Canadian Journal of Forest Research 29(1):95–105.
Vrsanska M, Voberkova S, Langer V, Palovcikova D, Moulick A, Adam V, Kopel P. 2016. Induction of Laccase, Lignin Peroxidase and Manganese Peroxidase Activities in White-Rot Fungi Using Copper Complexes. Molecules 21(11):1553.
Ward A, Dargusch P, Thomas S, Liu Y, Fulton EA. 2014. A global estimate of carbon stored in the world’s mountain grasslands and shrublands, and the implications for climate policy. Global Environmental Change 28:14–24.
Xu ZW, Yu GR, Zhang XY, Ge JP, He NP, Wang QF, Wang D. 2015. The variations in soil microbial communities, enzyme activities and their relationships with soil organic matter decomposition along the northern slope of Changbai Mountain. Applied Soil Ecology 86:19–29.
Zhao ZH, Liu GH, Mou NX, Xie YC, Xu ZR, Li Y. 2018. Assessment of Carbon Storage and Its Influencing Factors in Qinghai-Tibet Plateau. Sustainability 10(6):1864.
Zhu J, He X, Wu F, Yang W, Tan B. 2012. Decomposition of Abies faxoniana litter varies with freeze–thaw stages and altitudes in subalpine/alpine forests of southwest China. Scandinavian Journal of Forest Research 27(6):586–96.
Zifcakova L, Vetrovsky T, Howe A, Baldrian P. 2016. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environmental Microbiology 18(1):288–301.
Acknowledgements
We thank the anonymous reviewers and the editor for their insightful comments. This work was financially supported by projects from the National Natural Science Foundation of China (31570605), the Key Project of Sichuan Education Department (18ZA0393), National Key Research and Development Plan (2017YFC0505003) and Key Research and Development Project of Sichuan Province (18ZDYF0307). Haifeng Zheng acknowledges China Scholarship Council for supporting a Ph.D. program grant (201806910047). Petr Heděnec was supported by a Marie Curie European Fellowship (747824-AFOREST-H2020-MSCA-IF-2016/H2020-MSCA-IF-2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
HZ and YC conceived and designed the study with advice by YL. HZ, YC, YP, ZX, BT, LZ, LG, and LW performed the research. HZ, LV, and PH analysed the data; HZ led the writing of the first draft. LV, YL, and PH gave insightful suggestion for the improvement in the manuscript. All authors reviewed and approved the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, H., Chen, Y., Liu, Y. et al. Effects of Litter Quality Diminish and Effects of Vegetation Type Develop During Litter Decomposition of Two Shrub Species in an Alpine Treeline Ecotone. Ecosystems 24, 197–210 (2021). https://doi.org/10.1007/s10021-020-00512-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-020-00512-9