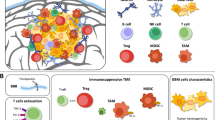
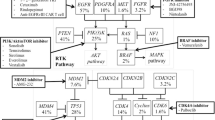
Abstract
Purpose of Review
Glioblastoma (GBM) is the most common malignant primary brain tumor, and the available treatment options are limited. This article reviews the recent preclinical and clinical investigations that seek to expand the repertoire of effective medical and radiotherapy options for GBM.
Recent Findings
Recent phase III trials evaluating checkpoint inhibition did not result in significant survival benefit. Select vaccine strategies have yielded promising results in early phase clinical studies and warrant further validation. Various targeted therapies are being explored but have yet to see breakthrough results. In addition, novel radiotherapy approaches are in development to maximize safe dose delivery.
Summary
A multitude of preclinical and clinical studies in GBM explore promising immunotherapies, targeted agents, and novel radiation modalities. Recent phase III trial failures have once more highlighted the profound tumor heterogeneity and diverse resistance mechanisms of glioblastoma. This calls for the development of biomarker-driven and personalized treatment approaches.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40.
Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma a randomized clinical trial. JAMA. 2015;314(23):2535–43.
Kurz SC, Wen PY. Quo Vadis—do immunotherapies have a role in glioblastoma? Curr Treat Options Neurol. 2018;20(5):14.
Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20(9):1100–9.
McGinnis GJ, Raber J. CNS side effects of immune checkpoint inhibitors: preclinical models, genetics and multimodality therapy. Immunotherapy. 2017;9(11):929–41.
Thakar MS, Kearl TJ, Malarkannan S. Controlling cytokine release syndrome to harness the full potential of CAR-based cellular therapy. Front Oncol. 2019;9:1529.
Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature Rev Cancer. 2016;16(5):275–87.
Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 2015;17(7):vii9–14.
• Omuro A, Vlahovic G, Lim M, Sahebjam S, Baehring J, Cloughesy T, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674–86 Early stage results demonstrated favorable toxicity and survivable profile in nivolumab with or without ipilumumab in recurrent GBM.
• Reardon DA, Omuro A, Brandes AA, Rieger J, Wick A, Sepulveda J, et al. OS10.3 randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro Oncol. 2017;19(suppl 3):iii21 Phase III results comparing nivolumab versus bevacizumab demonstrated no survival benefit in recurrent GBM.
Bristol-Myers Squibb Announces Phase 3 CheckMate −498 Study Did Not Meet Primary Endpoint of Overall Survival with Opdivo (nivolumab) Plus Radiation in Patients with Newly Diagnosed MGMT-Unmethylated Glioblastoma Multiforme | BMS Newsroom. Available from: https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-phase-3-checkmate-498-study-did. Accessed March 3, 2020.
Bristol-Myers Squibb Provides Update on Phase 3 Opdivo (nivolumab) CheckMate −548 Trial in Patients with Newly Diagnosed MGMT-Methylated Glioblastoma Multiforme | BMS Newsroom. Available from: https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-provides-update-phase-3-opdivo-nivolumab-. Accessed March 3, 2020.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13.
Bouffet E, Larouche V, Campbell BB, Merico D, De Borja R, Aronson M, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–11.
Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–86.
Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21(18):4062–72.
Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375:2561–9.
Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, et al. HER2-specific chimeric antigen receptor–modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3(8):1094–101.
• O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399):eaaa0984 Exploratory cohort of EGFRvIII CAR-T therapy highlights antigen escape as a possible reason for treatment failure.
•• Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37(9):1049–58 Novel approach to engineering EGFRvIII CAR-T cells to circumvent antigen escape demonstrated considerable promise in preclinical models.
Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–85.
Reardon DA, Desjardins A, Vredenburgh JJ, O’Rourke DM, Tran DD, Fink KL, et al. Rindopepimut with bevacizumab for patients with relapsed EGFRvIII-expressing glioblastoma (ReACT): results of a double-blind randomized phase II trial. Clin Cancer Res. 2020;26:1586–94. https://doi.org/10.1158/1078-CCR-18-1140.
Fenstermaker RA, Ciesielski MJ, Qiu J, Yang N, Frank CL, Lee KP, et al. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol Immunother. 2016;65(11):1339–52.
•• Ahluwalia MS, Reardon DA, Abad AP, Curry WT, Wong ET, Belal A, et al. SurVaxM with standard therapy in newly diagnosed glioblastoma: phase II trial update. J Clin Oncol. 2019;37(suppl 15):2016 Administration of a multi-peptide vaccine yielded favorable survival compared to historical controls, prompting a randomized, prospective evaluation that is expected to open to accrual in 2020.
Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–5.
•• Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–9 This article highlights that a personalized neoantigen-driven vaccines strategy is feasible in patients with glioblastoma and leads to appropriate intratumoral T cell response.
Peereboom DM, Nabors LB, Kumthekar P, Badruddoja MA, Fink KL, Lieberman FS, et al. Phase 2 trial of SL-701 in relapsed/refractory (r/r) glioblastoma (GBM): correlation of immune response with longer-term survival. J Clin Oncol. 2018;36(suppl 15):2058.
• Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16(1):142 Unblinded results from phase III trial of DCVax-L, an autologous dendritic cell vaccine.
Rahman M, Dastmalchi F, Karachi A, Mitchell D. The role of CMV in glioblastoma and implications for immunotherapeutic strategies. Oncoimmunology. 2018;8(1):e1514921.
Batich KA, Reap EA, Archer GE, Sanchez-Perez L, Nair SK, Schmittling RJ, et al. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin Cancer Res. 2017;23(8):1898–909.
•• Lassman AB, Reardon DA, Lee EQ, Iwamoto FM, Diaz-Mitoma F, Anderson DE, et al. Interim results of a phase I/IIa trial of a therapeutic CMV vaccine against recurrent glioblastoma (GBM). J Clin Oncol. 2019;37(suppl 15):2048 Interim results evaluating a peptide vaccine against CMV antigens demonstrated no safety concerns with undergoing phase IIa extension evaluating efficacy in additional subjects.
Wen PY, Reardon DA, Armstrong TS, Phuphanich S, Aiken RD, Landolfi JC, et al. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin Cancer Res. 2019;25(19):5799–807.
Cloughesy TF, Landolfi J, Vogelbaum MA, Ostertag D, Elder JB, Bloomfield S, et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-Oncology. 2018;20(10):1383–92.
Tocagen Reports Results of Toca 5 Phase 3 Trial in Recurrent Brain Cancer. Available from: https://www.prnewswire.com/news-releases/tocagen-reports-results-of-toca-5-phase-3-trial-in-recurrent-brain-cancer-300916705.html. Accessed March 3, 2020.
Brenner AJ, Peters KB, Vredenburgh J, Bokstein F, Blumenthal DT, Yust-Katz S, et al. Safety and efficacy of VB-111, an anticancer gene therapy, in patients with recurrent glioblastoma: results of a phase I/II study. Neuro-Oncology. 2019. https://doi.org/10.1093/neuonc/noz231.
Cloughesy TF, Brenner A, de Groot JF, Butowski NA, Zach L, Campian JL, et al. A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro-Oncology. 2019. https://doi.org/10.1093/neuonc/noz232.
Chiocca EA, Yu JS, Lukas RV, Solomon IH, Ligon KL, Nakashima H, et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Sci Transl Med. 2019;11(505):eaaw5680.
Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150–61.
Lang FF, Conrad C, Gomez-Manzano C, Alfred Yung WK, Sawaya R, Weinberg JS, et al. Phase I study of DNX-2401 (delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1319–427.
McLendon R, Friedman A, Bigner D, Van Meir EG, Brat DJ, Mastrogianakis GM, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8.
Brennan CW, Verhaak RGW, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–77.
Kelly WJ, Shah NJ, Subramaniam DS. Management of brain metastases in epidermal growth factor receptor mutant non-small-cell lung cancer. Front Oncol. 2018;8:208.
De Witt Hamer PC. Small molecule kinase inhibitors in glioblastoma: a systematic review of clinical studies. Neuro-Oncology. 2010;12(3):304–16.
Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, et al. BRAF inhibition in BRAFV600-mutant gliomas: results from the VE-BASKET study. J Clin Oncol. 2018;36(35):3477–84.
Wen P, Stein A, van den Bent M, De Greve J, Dietrich S, De Vos F, et al. ACTR-30. Updated efficacy and safety of dabrafenib plus trametinib in patients with recurrent/refractory BRAF V600E-mutated high-grade glioma (HGG) and low-grade glioma (LGG). Neuro Oncol. 2019;21(suppl 6):vi19–20.
Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019;30(suppl 8):vii23–30.
Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–55.
• Lombardi G, De Salvo GL, Brandes AA, Eoli M, Rudà R, Faedi M, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019;20(1):110–9 Phase II evaluation of a multi-kinase small molecule inhibitor demonstrated survival benefit over lomustine.
•• Alexander BM, Ba S, Berger MS, Berry DA, Cavenee WK, Chang SM, et al. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin Cancer Res. 2018;24(4):737–43 An ongoing platform trial designed to seamlessly evaluate multiple treatment regimens in newly diagnosed and recurrent GBM.
Chi AS, Tarapore RS, Hall MD, Shonka N, Gardner S, Umemura Y, et al. Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J Neuro-Oncol. 2019;145(1):97–105.
Ishizawa J, Zarabi SF, Davis RE, Halgas O, Nii T, Jitkova Y, et al. Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell. 2019;35(5):721–37.
Arrillaga-Romany I, Chi AS, Allen JE, Oster W, Wen PY, Batchelor TT. A phase 2 study of the first imipridone ONC 201, a selective DRD 2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget. 2017;8(45):79298–304.
Taylor JW, Parikh M, Phillips JJ, James CD, Molinaro AM, Butowski NA, et al. Phase-2 trial of palbociclib in adult patients with recurrent RB1-positive glioblastoma. J Neuro-Oncol. 2018;140(2):477–83.
•• Tien AC, Li J, Bao X, Derogatis A, Kim S, Mehta S, et al. A phase 0 trial of ribociclib in recurrent glioblastoma patients incorporating a tumor pharmacodynamic- and pharmacokinetic-guided expansion cohort. Clin Cancer Res. 2019;25(19):5777–86 This article highlights the phase 0 clinical study approach in the development of novel agents to assure appropriate penetration across the blood brain barrier and pharmacodynamic activity within the tumor before proceeding to early phase clinical development.
Raub TJ, Wishart GN, Kulanthaivel P, Staton BA, Ajamie RT, Sawada GA, et al. Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab Dispos. 2015;43(9):1360–71.
Anders CK, Le Rhun E, Bachelot TD, Yardley DA, Awada A, Conte PF, et al. A phase II study of abemaciclib in patients (pts) with brain metastases (BM) secondary to HR+, HER2- metastatic breast cancer (MBC). J Clin Oncol. 2019;37(suppl 15):1017.
•• Alexander BM, Trippa L, Gaffey S, Arrillaga-Romany IC, Lee EQ, Rinne ML, et al. Individualized screening trial of innovative glioblastoma therapy (INSIGhT): a Bayesian adaptive platform trial to develop precision medicines for patients with glioblastoma. JCO Precis Oncol. 2019. https://doi.org/10.1200/PO.18.00071An ongoing platform trial designed to evaluate precision medicine approaches in GBM.
Goel S, Decristo MJ, Watt AC, Brinjones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–5.
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708.
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22.
Neyns B, Sadones J, Joosens E, Bouttens F, Verbeke L, Baurain JF, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20(9):1596–603.
van den Bent M, Eoli M, Sepulveda JM, Smits M, Walenkamp A, Frenel J-S, et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFRamplified glioblastoma. Neuro-Oncology. 2019. https://doi.org/10.1093/neuonc/noz222.
Phillips AC, Boghaert ER, Vaidya KS, Falls HD, Mitten MJ, Devries PJ, et al. Characterization of ABBV-221, a tumor-selective EGFR-targeting antibody drug conjugate. Mol Cancer Ther. 2018;17:795–806.
Palanichamy K, Chakravarti A. Combining drugs and radiotherapy: from the bench to the bedside. Curr Opin Neurol. 2009;22(5):625–32.
Dungey FA, Löser DA, Chalmers AJ. Replication-dependent radiosensitization of human glioma cells by inhibition of poly(ADP-ribose) polymerase: mechanisms and therapeutic potential. Int J Radiat Oncol Biol Phys. 2008;72(4):1188–97.
Chalmers AJ, Short S, Watts C, Herbert C, Morris A, Stobo J, et al. Phase I clinical trials evaluating olaparib in combination with radiotherapy (RT) and/or temozolomide (TMZ) in glioblastoma patients: results of OPARATIC and PARADIGM phase I and early results of PARADIGM-2. J Clin Oncol. 2018;36(suppl 15):2018.
Piotrowski A, Puduvalli V, Wen P, Campian J, Colman H, Pearlman M, et al. ACTR-39. Pamiparib in combination with radiation therapy (RT) and/or temozolomide (TMZ) in patients with newly diagnosed or recurrent/refractory (R/R) glioblastoma (GBM); a phase 1B/2 study update. Neuro Oncol. 2019;21(suppl 6):vi21–2.
Khasraw M, McDonald KL, Rosenthal M, Lwin Z, Ashley DM, Wheeler H, et al. A randomized phase II trial of veliparib (V), radiotherapy (RT) and temozolomide (TMZ) in patients (pts) with unmethylated MGMT (uMGMT) glioblastoma (GBM). J Clin Oncol. 2019;37(suppl 15):2011.
Ihara M, Ashizawa K, Shichijo K, Kudo T. Expression of the DNA-dependent protein kinase catalytic subunit is associated with the radiosensitivity of human thyroid cancer cell lines. J Radiat Res. 2019;60(2):171–7.
Weterings E, Gallegos AC, Dominick LN, Cooke LS, Bartels TN, Vagner J, et al. A novel small molecule inhibitor of the DNA repair protein Ku70/80. DNA Repair (Amst). 2016;43:98–106.
Yan D, Ng WL, Zhang X, Wang P, Zhang Z, Mo Y-Y, et al. Targeting DNA-PKcs and ATM with miR-101 sensitizes tumors to radiation. PLoS One. 2010;5(7):e11397.
Munster P, Mita M, Mahipal A, Nemunaitis J, Massard C, Mikkelsen T, et al. First-in-human phase i study of a dual mTOR kinase and DNA-PK inhibitor (CC-115) in advanced malignancy. Cancer Manag Res. 2019;11:10463–76.
Carruthers R, Ahmed SU, Strathdee K, Gomez-Roman N, Amoah-Buahin E, Watts C, et al. Abrogation of radioresistance in glioblastoma stem-like cells by inhibition of ATM kinase. Mol Oncol. 2015;9(1):192–203.
Ahmed SU, Carruthers R, Gilmour L, Yildirim S, Watts C, Chalmers AJ. Selective inhibition of parallel DNA damage response pathways optimizes radiosensitization of glioblastoma stem-like cells. Cancer Res. 2015;75(20):4416–28.
Biddlestone-Thorpe L, Sajjad M, Rosenberg E, Beckta JM, Valerie NCK, Tokarz M, et al. ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin Cancer Res. 2013;19(12):3189–200.
Barani IJ, Larson DA. Radiation therapy of glioblastoma. Cancer Treat Res. 2015;163:49–73.
Patyal B. Dosimetry aspects of proton therapy. Technol Cancer Res Treat. 2007;6(4 suppl):17–23.
Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–80.
Mizumoto M, Yamamoto T, Ishikawa E, Matsuda M, Takano S, Ishikawa H, et al. Proton beam therapy with concurrent chemotherapy for glioblastoma multiforme: comparison of nimustine hydrochloride and temozolomide. J Neuro-Oncol. 2016;130(1):165–70.
Mizumoto M, Yamamoto T, Takano S, Ishikawa E, Matsumura A, Ishikawa H, et al. Long-term survival after treatment of glioblastoma multiforme with hyperfractionated concomitant boost proton beam therapy. Pract Radiat Oncol. 2015;5(1):e9–16.
• Amelio D, Scartoni D, Farace P, Widesott L, Vennarini S, Fellin F, et al. P01.084 re-irradiation in recurrent glioblastoma: proton therapy with or without chemotherapy. Neuro Oncol. 2018;20(suppl 3):ii249 Preliminary analysis demonstrated a favorable safety profile of proton re-irradiation with concurrent chemotherapy in recurrent GBM.
Nakano T, Suzuki Y, Ohno T, Kato S, Suzuki M, Morita S, et al. Carbon beam therapy overcomes the radiation resistance of uterine cervical cancer originating from hypoxia. Clin Cancer Res. 2006;1(12):2185–90.
Chiblak S, Tang Z, Campos B, Gal Z, Unterberg A, Debus J, et al. Radiosensitivity of patient-derived glioma stem cell 3-dimensional cultures to photon, proton, and carbon irradiation. Int J Radiat Oncol Biol Phys. 2016;95(1):112–9.
Malouff TD, Peterson JL, Mahajan A, Trifiletti DM. Carbon ion radiotherapy in the treatment of gliomas: a review. J Neuro-Oncol. 2019;145(2):191–9.
Vogin G, Wambersie A, Koto M, Ohno T, Uhl M, Fossati P, et al. A step towards international prospective trials in carbon ion radiotherapy: investigation of factors influencing dose distribution in the facilities in operation based on a case of skull base chordoma. Radiat Oncol. 2019;14(1):24.
Combs SE, Burkholder I, Edler L, Rieken S, Habermehl D, Jäkel O, et al. Randomised phase I/II study to evaluate carbon ion radiotherapy versus fractionated stereotactic radiotherapy in patients with recurrent or progressive gliomas: the CINDERELLA trial. BMC Cancer. 2010;10:1–8.
• Combs SE, Bernhardt D, Adeberg S, Herfarth KK, Unterberg A, Wick W, et al. Carbon ion reirradiaton for patients with malignant gliomas: toxicity and first results of the prospective dose-escalation phase I/II CINDERELLA trial. J Clin Oncol. 2019;37(suppl 15):2059 A prospective phase I/II study found favorable survival and toxicity results following carbon ion re-irradiation in recurrent GBM.
Weidlich GA, Bodduluri M, Achkire Y, Lee C, Adler JR. Characterization of a novel 3 megavolt linear accelerator for dedicated intracranial stereotactic radiosurgery. Cureus. 2019;11(3):e4275.
Jenkins CH, Kahn R, Weidlich GA, Adler JR. Radiosurgical treatment verification using removable megavoltage radiation detectors. Cureus. 2017;9(11):e1889.
Adler JR, Schweikard A, Achkire Y, Blanck O, Bodduluri M, Ma L, et al. Treatment planning for self-shielded radiosurgery. Cureus. 2017;9(9):e1663.
Podgorsak EB, Bruce Pace G, Olivier A, Pla M, Souhami L. Radiosurgery with high energy photon beams: a comparison among techniques. Int J Radiat Oncol Biol Phys. 1989;16(3):857–65.
Ferreira C, Alaei P, Chen C, Reynolds M, Sterling D, Dusenbery K. RTHP-32. First experience with GammaTile permanent implants for recurrent brain tumors.
Nakaji P, Youssef Emad, Dardis C, Smih K, Pinnaduwage D, et al. Surgically targeted radiation therapy: a prospective trial in 79 recurrent, previously irradiated intracranial neoplasms. American Association of Neurological Surgeons Annual Scientific Meeting. 2019. Abstract 207.
Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6(245):245ra93.
Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci U S A. 2019;166(22):10943–51.
Simmons DA, Lartey FM, Schüler E, Rafat M, King G, Kim A, et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother Oncol. 2019;139:4–10.
Montay-Gruel P, Petit B, Bochud F, Favaudon V, Bourhis J, Vozenin MC. PO-0799: normal brain, neural stem cells and glioblastoma responses to FLASH radiotherapy. Radiother Oncol. 2015;115(suppl 1):S400–1.
• Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019;139:18–22 The first case of FLASH radiotherapy treatment demonstrated a durable response in multiresistant T-cell cutaneous lymphoma.
Venkatesulu BP, Sharma A, Pollard-Larkin JM, Sadagopan R, Symons J, Neri S, et al. Ultra high dose rate (35 Gy/sec) radiation does not spare the normal tissue in cardiac and splenic models of lymphopenia and gastrointestinal syndrome. Sci Rep. 2019;9:17180.
Wilson JD, Hammond EM, Higgins GS, Petersson K. Ultra-high dose rate (FLASH) radiotherapy: silver bullet or fool’s gold? Front Oncol. 2020;9:1563.
Kamath AA, Akbari SHA. Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: safety, efficacy, and outcomes. Neurosurgery. 2019;84(4):836–43.
Sloan AE, Ahluwalia MS, Valerio-Pascua J, Manjila S, Torchia MG, Jones SE, et al. Results of the NeuroBlate system first-in-humans phase I clinical trial for recurrent glioblastoma. J Neurosurg. 2013;118(6):1202–19.
Draaisma K, Chatzipli A, Taphoorn M, Kerkhof M, Weyerbrock A, Sanson M, et al. Molecular evolution of IDH wild-type glioblastomas treated with standard of care affects survival and design of precision medicine trials: a report from the EORTC 1542 study. J Clin Oncol. 2010;38(1):81–99.
Barthel FP, Johnson KC, Varn FS, Moskalik AD, Tanner G, Kocakavuk E, et al. Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019;576(7785):112–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
E. Liu has nothing to disclose.
Dr. Sulman reports grants and personal fees from AbbVie, grants, personal fees and non-financial support from Novocure, personal fees and non-financial support from Merck, personal fees and non-financial support from BrainLab, personal fees and non-financial support from Blue Earth Diagnositics, personal fees and non-financial support from Physician’s Education Resource, personal fees and non-financial support from Zai Lab, outside the submitted work.
Dr. Wen reports grants from Agios, grants from Astra Zenenca, grants from Karyopharm, grants from Sanofi-Aventis, grants from Vascular Biogenics, other from Vascular Biogenics, other from Merck, grants from Celgene, grants from Eli Lily, grants from Kazia, grants from MediciNova, grants from Novartis, grants from Oncoceutics, grants from VBI Vaccines, personal fees from Astra Zeneca, personal fees from Bayer, personal fees from Blue Earth Diagnostics, personal fees from Karyopharm, personal fees from Tocagen, personal fees from Integral Health, personal fees from Prime Oncology, personal fees from Imvax, personal fees from Elevate Bio, and personal fees from QED, outside the submitted work.
Dr. Kurz reports that she is the principal investigator on two immunotherapy studies that have been mentioned in this work (NCT02968940, NCT03367715).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-Oncology
Rights and permissions
About this article
Cite this article
Liu, E.K., Sulman, E.P., Wen, P.Y. et al. Novel Therapies for Glioblastoma. Curr Neurol Neurosci Rep 20, 19 (2020). https://doi.org/10.1007/s11910-020-01042-6
Published:
DOI: https://doi.org/10.1007/s11910-020-01042-6