Summary
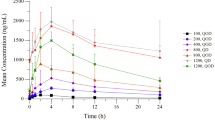
Background PF-06840003 is a highly selective indoleamine 2, 3-dioxygenase (IDO1) inhibitor with antitumor effects in preclinical models. This first-in-human phase 1 study evaluated safety, pharmacokinetics/pharmacodynamics, and preliminary efficacy in recurrent malignant glioma to determine the maximum tolerated dose (MTD) or recommended phase 2 dose (RP2D). Methods Patients (N = 17) received oral PF-06840003 in four dose-escalation groups: 125 mg once-daily (QD; n = 2); 250 mg QD (n = 4); 250 mg twice-daily (BID; n = 3); 500 mg BID (n = 8). A modified toxicity probability interval method determined the MTD. Results Four patients experienced serious adverse events (SAEs); one with treatment-related SAEs (grade 4 alanine and aspartate aminotransferase elevations). The dose-limiting toxicity (DLT) rate at 500 mg BID was 12.5% (n = 1/8); the MTD was not reached. Following PF-06840003 dosing, median time to maximum plasma concentration for the active enantiomer PF-06840002 was 1.5–3.0 hr and mean elimination half-life was 2 to 4 hr (Cycle 1 Day 1). Urinary recovery of PF-06840002 was low (< 1%). At 500 mg BID, maximum mean percentage inhibition of 13C10 kynurenine vs endogenous kynurenine was 75% vs 24%. PF-06840002 CSF-to-plasma ratio was 1.00. Disease control occurred in eight patients (47%). Mean duration of stable disease (SD) was 32.1 (12.1–72.3) weeks. Two patients with SD discontinued the study at 450 and 561 days and continued PF-06840003 on compassionate use. Conclusion PF‑06840003 up to 500 mg BID was generally well tolerated with evidence of a pharmacodynamic effect and durable clinical benefit in a subset of patients with recurrent malignant glioma. ClinicalTrials.gov, NCT02764151, registered April 2016.



Similar content being viewed by others
Data availability
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices: (1) for indications that have been approved in the US and/or EU; or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
Ostrom QT, Bauchet L, Davis FG et al (2014) The epidemiology of glioma in adults: a “state of the science” review. Neuro-Oncology 16:896–913. https://doi.org/10.1093/neuonc/nou087
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 15(suppl 2):ii1-i56. https://doi.org/10.1093/neuonc/not151
Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G (2014) High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(suppl 3):iii93-i101. https://doi.org/10.1093/annonc/mdu050
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. https://doi.org/10.1016/s1470-2045(09)70025-7
Lancellotti S, Novarese L, De Cristofaro R (2011) Biochemical properties of indoleamine 2,3-dioxygenase: from structure to optimized design of inhibitors. Curr Med Chem 18:2205–2214. https://doi.org/10.2174/092986711795656108
Opitz CA, Litzenburger UM, Sahm F et al (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478:197–203. https://doi.org/10.1038/nature10491
Mellor AL, Munn DH (2004) IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4:762–774. https://doi.org/10.1038/nri1457
Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9:1269–1274. https://doi.org/10.1038/nm934
Munn DH, Mellor AL (2013) Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 34:137–143. https://doi.org/10.1016/j.it.2012.10.001
van Baren N, Van den Eynde BJ (2015) Tumoral immune resistance mediated by enzymes that degrade tryptophan. Cancer Immunol Res 3:978–985. https://doi.org/10.1158/2326-6066.Cir-15-0095
Okamoto A, Nikaido T, Ochiai K et al (2005) Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res 11:6030–6039. https://doi.org/10.1158/1078-0432.Ccr-04-2671
Brandacher G, Perathoner A, Ladurner R et al (2006) Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res 12:1144–1151. https://doi.org/10.1158/1078-0432.Ccr-05-1966
Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H (2013) Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery 72:1031–1038; discussion 1038–1039. https://doi.org/10.1227/NEU.0b013e31828cf945
Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, Tobias AL, Han Y, Lesniak MS (2012) IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 18:6110–6121. https://doi.org/10.1158/1078-0432.Ccr-12-2130
Gomes B, Driessens G, Bartlett D et al (2018) Characterization of the selective indoleamine 2,3-dioxygenase-1 (IDO1) catalytic inhibitor EOS200271/PF-06840003 supports IDO1 as a critical resistance mechanism to PD-(L)1 blockade therapy. Mol Cancer Ther 17:2530–2542. https://doi.org/10.1158/1535-7163.Mct-17-1104
Crosignani S, Bingham P, Bottemanne P et al (2017) Discovery of a novel and selective indoleamine 2,3-dioxygenase (IDO-1) inhibitor 3-(5-Fluoro-1H-indol-3-yl)pyrrolidine-2,5-dione (EOS200271/PF-06840003) and its characterization as a potential clinical candidate. J Med Chem 60:9617–9629. https://doi.org/10.1021/acs.jmedchem.7b00974
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280. https://doi.org/10.1200/jco.1990.8.7.1277
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in Neuro-Oncology Working Group. J Clin Oncol 28:1963–1972. https://doi.org/10.1200/jco.2009.26.3541
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 54:457–481
Beatty GL, O’Dwyer PJ, Clark J et al (2017) First-in-human phase I study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin Cancer Res 23:3269–3276. https://doi.org/10.1158/1078-0432.Ccr-16-2272
Siu LL, Gelmon K, Chu Q et al (2017) Abstract CT116: BMS-986205, an optimized indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, is well tolerated with potent pharmacodynamic (PD) activity, alone and in combination with nivolumab (nivo) in advanced cancers in a phase 1/2a trial. Cancer Res 77:CT116. https://doi.org/10.1158/1538-7445.AM2017-CT116
Funding
This study was sponsored by Pfizer. Medical writing support was provided by David Sunter, PhD, CMPP, of Engage Scientific Solutions and funded by Pfizer. AB Lassman was supported in part by Voices Against Brain Cancer, the William Rhodes and Louise Tilzer-Rhodes Center for Glioblastoma at NewYork-Presbyterian Hospital, and grants P30CA013696 and UG1CA189960 from the National Cancer Institute (NCI). The content is solely the responsibility of the authors and does not necessarily represent the views of the NCI/NIH.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing of this manuscript, the analysis and interpretation of data, and provided critical manuscript revision and approval of final content.
Corresponding author
Ethics declarations
Conflict of interest
DA Reardon declares research support (paid to DFCI): Acerta Phamaceuticals, Agenus, Celldex, EMD Serono, Incyte, Inovio, Midatech, Omniox, and Tragara; Advisory/consultation (paid to Dr Reardon): AbbVie, Advantagene, Agenus, Amgen, Bayer, Bristol-Myers Squibb, Celldex, DelMar, EMD Serono, Genentech/Roche, Inovio, Merck, Merck KGaA, Monteris, Novocure, Oncorus, Oxigene, Regeneron, Stemline, and Taiho Oncology Inc; Honoraria (paid to Dr Reardon): AbbVie, Advantagene, Agenus, Bristol-Myers Squibb, Celldex, EMD Serono, Genentech/Roche, Inovio, Merck, Merck KGaA, Monteris, Novocure, Oncorus, Oxigene, Regeneron, Stemline, and Taiho Oncology Inc; A Desjardins declares research research support (paid to Duke): Orbus Therapeutics, Genentech/Roche, Triphase Accelerator, Symphogen A/S; has served as consultant/participated in advisory boards (paid to Dr Desjardins: Orbus Therapeutics and Istari Oncology; as stock options in Istari Oncology; and patent on the use of the oncolytic poliovirus for the treatment of solid tumors; T Cloughesy declares research support from AstraZeneca, honoraria from Roche for serving on the Speakers Bureau, has served as a consultant/participated in advisory boards for VBL, Bayer, GW Pharma, Tocagen, Del Mar, Amgen, QED, Merck, Karyopharm, Odonate, Pascal Biosciences, Agios, is a Board member for Global Coalition for Adaptive Research 501 ©3, has stock options with Notable Labs, has a provisional patent application for compositions and methods for treating cancer; O Rixe declares research support from: Kyowa/Kirin, Oxford Biotherapeutics, Nanobiotix, IMV Inc, Newlink, Seattle Genetics, has served on the advisory committee for Kyowa/Kirin, Bexion, and is a stock shareholder in Bexion; S Alekar is an employee of Pfizer and hold stock in Pfizer, JH Williams is an employee of Pfizer and hold stock in Pfizer, CT Taylor is an employee of Pfizer and hold stock in Pfizer, R Li is an employee of Pfizer and hold stock in Pfizer; AB Lassman declares grants and non-financial support from Pfizer, during the conduct of the study; personal fees and non-financial support from Bioclinica as an expert blinded independent reviewer of clinical and imaging data for a BMS-sponsored trial, grants, personal fees and non-financial support from Karyopharm, personal fees from Sapience, personal fees from Magnolia Innovation, personal fees and non-financial support from Guidepoint Global, personal fees and non-financial support from Abbott Molecular, grants, personal fees and non-financial support from QED Therapeutics, personal fees from GLG, personal fees from Olson Research Group, personal fees from Biostrategies Group, personal fees and non-financial support from Forma, personal fees from Clarion Health Care Consulting, grants, personal fees and non-financial support from Bayer, personal fees from Defined Health, personal fees from Research America, personal fees from Advanced Focus, personal fees from Expert Connect, personal fees from Elsevier, grants, personal fees and non-financial support from Orbus, personal fees and non-financial support from Northwest Biotherapeutics, grants, personal fees and non-financial support from AbbVie, personal fees from Bionest Partners, personal fees from Antheneum, personal fees from DecioBio, personal fees from Connected Research and Consulting, personal fees from Health Advisors Bureau/Medefield, grants, personal fees and non-financial support from Agios, personal fees from RICCA Group, personal fees from Medsurvey, personal fees from Focus Forward Incentives, personal fees from WebMD, personal fees from Celgene, personal fees from prIME Oncology, personal fees from marketplusresearch.com, personal fees from Market Strategies International, personal fees from MSI Survey, personal fees from Cortice Biosciences, grants, personal fees and non-financial support from Kadmon, personal fees from Schlesinger Associates, personal fees and non-financial support from Novocure, personal fees and non-financial support from Physicians' Education Resource/Chemotherapy Foundation Symposium, grants and non-financial support from Genentech/Roche, grants and non-financial support from Amgen, grants and non-financial support from Millienium, grants and non-financial support from Celldex, grants and non-financial support from Novartis, non-financial support from Keryx as legacy to Aeterna Zentaris, grants and non-financial support from VBI Vaccines, grants and non-financial support from Beigene, grants and non-financial support from Oncoceutics, non-financial support from Tocagen, outside the submitted work.
Ethical approval
Research involving human participants: The study was approved by the institutional review board or independent ethics committee of each participating center and followed the Declaration of Helsinki and International Council for Harmonization Good Clinical Practice guidelines.
Informed consent
All subjects or legally authorized representatives provided written informed consent.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 223 KB)
Rights and permissions
About this article
Cite this article
Reardon, D.A., Desjardins, A., Rixe, O. et al. A phase 1 study of PF-06840003, an oral indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor in patients with recurrent malignant glioma. Invest New Drugs 38, 1784–1795 (2020). https://doi.org/10.1007/s10637-020-00950-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00950-1