Summary
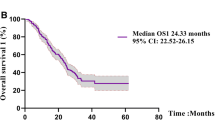
Osimertinib is a molecularly targeted agent used to treat non-small cell lung cancer (NSCLC) patients with an epidermal growth factor receptor (EGFR) T790M mutation. However, its efficacy and safety profile when patients have poor performance status (PS) is unknown. Therefore, we conducted an open-label, multi-center, single-arm phase II study to evaluate its efficacy and safety in EGFR T790M mutation-positive NSCLC patients with Eastern Cooperative Oncology Group PS scores of between 2 and 4. Patients received 80 mg of osimertinib once daily. Our primary endpoint was progression-free survival. Eighteen patients were enrolled between June 2017 and November 2018. The median age was 77 years (range: 55–85 years). Ten, six, and two patients had PS scores of 2, 3, and 4, respectively. All patients had adenocarcinoma with common EGFR mutations and had been treated with first- or second-generation EGFR- tyrosine kinase inhibitors previously. The overall median progression-free survival was 7.0 months (90% confidence interval: 5.5–8.9 months). The overall response rate and median overall survival were 53% and 12.7 months, respectively. Moreover, improved PS scores were observed in 72% of the patients. Although the incidence of grade 3 adverse events was low, with no grade 4 or 5 events observed, three patients required treatment cessation due to the development of interstitial lung disease. Osimertinib therapy could be beneficial for EGFR T790M mutation-positive advanced NSCLC patients with poor PS. This trial was registered with the Japan Registry of Clinical Trials on March 12, 2019 (trial no. jRCT1041180081).




Similar content being viewed by others
References
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small-cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83:584–594
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139
Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF (2005) Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97:339–346
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M, West Japan Oncology Group (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T, North-East Japan Study Group (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12:735–742
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, de Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L, Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246
Sequist LV, Yang JCH, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31:3327–3334
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, Xu CR, Massey D, Kim M, Shi Y, Geater SL (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15:213–222
Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, Riely GJ (2013) Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 19:2240–2247
Jänne PA, Yang JCH, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, Haggstrom D, Felip E, Kim JH, Frewer P, Cantarini M, Brown KH, Dickinson PA, Ghiorghiu S, Ranson M (2015) AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. New Engl J Med 372:1689–1699
Mok TS, Wu Y-L, Ahn MJ et al (2016) Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. New Engl J Med 376:629–640
Nakashima K, Kimura M, Akamatsu H, Daga H, Imai H, Taira T, Ko R, Hisamatsu Y, Nishino K, Sugimoto T, Miyashita Y, Takahashi T, et al (2019) Osimertinib for patients with EGFR T790M mutation-positive non-small-cell lung cancer and a poor performance status. Jpn J Clin Oncol 49:671–675
Lilenbaum R, Herndon JE, List MA et al (2005) Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 23:190–196
Lilenbaum R, Axelrod R, Thomas S, Dowlati A, Seigel L, Albert D, Witt K, Botkin D (2008) Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 26:863–869
Langer C, Li S, Schiller J, Tester W, Rapoport BL, Johnson DH, Eastern Cooperative Oncology Group (2007) Randomized phase II trial of paclitaxel plus carboplatin or gemcitabine plus cisplatin in eastern cooperative oncology group performance status 2 non-small-cell lung cancer patients: ECOG 1599. J Clin Oncol 25:418–423
Saito H, Nakagawa K, Takeda K, Iwamoto Y, Ando M, Maeda M, Katakami N, Nakano T, Kurata T, Fukuoka M (2012) Randomized phase II study of carboplatin-paclitaxel or gemcitabine-vinorelbine in patients with advanced nonsmall cell lung cancer and a performance status of 2: West Japan thoracic oncology group 0004. Am J Clin Oncol 35:58–63
Brookmeyer R, Crowley JJ (1982) A confidence interval for the median survival time. Biometrics 38:29–41
Inoue A, Saijo Y, Maemondo M, Gomi K, Tokue Y, Kimura Y, Ebina M, Kikuchi T, Moriya T, Nukiwa T (2003) Severe acute interstitial pneumonia and gefitinib. Lancet 361:137–139
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS (2018) Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. New Engl J Med 378:113–125
Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, Ando M, Yamazaki K, Saijo Y, Gemma A, Miyazawa H, Tanaka T, Ikebuchi K, Nukiwa T, Morita S, Hagiwara K, North East Japan Gefitinib Study Group (2009) First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 27:1394–1400
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Author information
Authors and Affiliations
Contributions
Kazuhisa Nakashima (Corresponding Author): creating the study protocol, recruitment of patients, and writing the manuscript; Yuichi Ozawa: recruitment of patients and reviewing the manuscript; Haruko Daga: recruitment of patients and reviewing the manuscript; Hisao Imai: recruitment of patients and reviewing the manuscript; Motohiro Tamiya: recruitment of patients and reviewing the manuscript; Takaaki Tokito: recruitment of patients and reviewing the manuscript; Takahisa Kawamura: recruitment of patients and reviewing the manuscript; Hiroaki Akamatsu: recruitment of patients and reviewing the manuscript; Yuko Tsuboguchi: recruitment of patients and reviewing the manuscript; Toshiaki Takahashi: recruitment of patients and reviewing the manuscript; Nobuyuki Yamamoto: recruitment of patients and reviewing the manuscript; Keita Mori (Primary biostatistician of the study): creating the study protocol, statistical analysis, and reviewing the manuscript; Haruyasu Murakami: creating the study protocol, recruitment of patients, and writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Kazuhisa Nakashima declares that he has no conflict of interest. Yuichi Ozawa had received personal fees from AstraZeneca, Boehringer Ingelheim, and Chugai Pharma. Haruko Daga had received personal fees from Boehringer Ingelheim, Chugai Pharma, and Ono Pharmaceutical, and grants from Astella, Pfizer, and Taiho Pharmaceutical. Hisao Imai declares that he has no conflict of interest. Motohiro Tamiya had received personal fees from Asahi Kasei Pharmaceutical, AstraZeneca, Chugai Pharma, Eli Lilly, MSD, and Taiho Pharmaceutical, and personal fees and grants from Boehringer Ingelheim, Bristol-Myers Squibb, and Ono Pharmaceutical. Takaaki Tokito had received personal fees from AstraZeneca, Boehringer Ingelheim, and Chugai Pharma. Takahisa Kawamura declares that he has no conflict of interest. Hiroaki Akamatsu had received personal fees from AstraZeneca, Boehringer Ingelheim, Chugai Pharma, and Pfizer. Yuko Tsuboguchi declares that he has no conflict of interest. Toshiaki Takahashi had received personal fees from Boehringer Ingelheim and Roche Diagnostics K.K., grants from Japan Agency for Medical Research and Development, and personal fees and grants from AstraZeneca, Chugai Pharma, Eli Lilly, MSD, Ono Pharmaceutical, and Pfizer. Nobuyuki Yamamoto had received personal fees from AstraZeneca, and personal fees and grants from Boehringer Ingelheim and Chugai Pharma. Keita Mori declares that he has no conflict of interest. Haruyasu Murakami had received personal fees from Bristol-Myers Squibb, Ono Pharmaceutical, and MSD, grants from Abbvie, Daiichi Sankyo, and IQvia, and personal fees and grants from AstraZeneca, Chugai Pharma, Eli Lilly, Taiho Pharmaceutical, and Takeda.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board and the 1964 Helsinki declaration and its later amendments or with comparable ethical standards. This trial was registered with the Japan Registry of Clinical Trials on March 12, 2019 (trial no. jRCT1041180081).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakashima, K., Ozawa, Y., Daga, H. et al. Osimertinib for patients with poor performance status and EGFR T790M mutation-positive advanced non-small cell lung cancer: a phase II clinical trial. Invest New Drugs 38, 1854–1861 (2020). https://doi.org/10.1007/s10637-020-00943-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00943-0