Summary
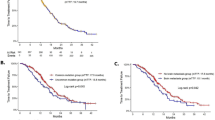
Introduction Afatinib is used to treat patients with advanced non-small cell lung cancer (NSCLC) harboring common EGFR mutations; however, the clinicopathological factors that predict this drug’s effectiveness in real-world settings remain unclear. We therefore evaluated the effectiveness of afatinib in such patients and assessed potential prognostic factors. Methods We retrospectively investigated patients with NSCLC who received first-line afatinib between July 2014 and August 2018. Variables (including sex, age, performance status, neutrophil-to-lymphocyte ratio, EGFR genotype, smoking status, clinical stage prior to treatment [stage IV vs.. postoperative recurrence], presence or absence of brain metastases, body surface area, any afatinib dose reductions, and afatinib starting dose [40 vs.. 20 or 30 mg]) were subjected to a Cox proportional hazards regression model to estimate progression-free survival (PFS). Results Forty-eight patients with a median age of 67 years were included; the objective response rate was 62.5% (30 patients). The median PFS was 14.1 months; the PFS periods were 11.8 and 15.9 months for patients receiving 40 mg versus 20–30 mg of afatinib (P = 0.41), respectively, and were 14.5 and 13.8 months for patients who required afatinib dose reduction and those who did not, respectively (P = 0.80). The PFS tended to be longer in patients without brain metastases (albeit not significantly). Ultimately, no significant predictive values for PFS were identified. Conclusions Afatinib is effective for patients with NSCLC harboring common EGFR mutations irrespective of their clinicopathological backgrounds. A direct comparison of afatinib and osimertinib in treatment-naïve patients is warranted to determine the optimal standard of care.



Similar content being viewed by others
References
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C et al (2017) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 3:524–548. https://doi.org/10.1001/jamaoncol.2016.5688
Siegel R, DeSantis C, Virgo K et al (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 62:220–241. https://doi.org/10.3322/caac.21149
Bria E, Milella M, Cuppone F et al (2011) Outcome of advanced NSCLC patients harboring sensitizing EGFR mutations randomized to EGFR tyrosine kinase inhibitors or chemotherapy as first-line treatment: a meta-analysis. Ann Oncol 22:2277–2285. https://doi.org/10.1093/annonc/mdq742
Petrelli F, Borgonovo K, Cabiddu M, Barni S (2012) Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non–small-cell lung cancer: a meta-analysis of 13 randomized trials. Clin Lung Cancer 13:107–114. https://doi.org/10.1016/j.cllc.2011.08.005
Paez JG, Jänne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500. https://doi.org/10.1126/science.1099314
Miyawaki M, Naoki K, Yoda S et al (2017) Erlotinib as second- or third-line treatment in elderly patients with advanced non-small cell lung cancer: Keio Lung Oncology Group Study 001 (KLOG001). Mol Clin Oncol 6:409–414. https://doi.org/10.3892/mco.2017.1154
Ramalingam AA, Vansteenkiste J, Planchard D et al (2020) Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382:41–50. https://doi.org/10.1056/NEJMoa1913662
Wang J, Wang B, Chu H, Yao Y (2016) Intrinsic resistance to EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer with activating EGFR mutations. Onco Targets Ther 9:3711–3726. https://doi.org/10.2147/OTT.S106399
Igawa S, Sasaki J, Otani S, Ishihara M, Takakura A, Katagiri M, Masuda N (2015) Impact of smoking history on the efficacy of gefitinib in patients with non-small cell lung cancer harboring activating epidermal growth factor receptor mutations. Oncology 89:275–280. https://doi.org/10.1159/000438703
Nishinarita N, Igawa S, Kasajima M et al (2018) Smoking history as a predictor of epidermal growth factor receptor tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring EGFR mutations. Oncology 95:109–115. https://doi.org/10.1159/000488594
Ono T, Igawa S, Kurahayashi S et al (2020) Impact of neutrophil-to-lymphocyte ratio in patients with EGFR-mutant NSCLC treated with tyrosine kinase inhibitors. Invest New Drugs. https://doi.org/10.1007/s10637-020-00919-0
Igawa S, Kasajima M, Ishihara M et al (2014) Evaluation of gefitinib efficacy according to body surface area in patients with non-small cell lung cancer harboring an EGFR mutation. Cancer Chemother Pharmacol 74:939–946. https://doi.org/10.1007/s00280-014-2570-1
Ono T, Igawa S, Ozawa T et al (2019) Evaluation of osimertinib efficacy according to body surface area and body mass index in patients with non-small cell lung cancer harboring an EGFR mutation: A prospective observational study. Thorac Cancer 10:880–889. https://doi.org/10.1111/1759-7714.13018
Du Bois D, Du Bois EF (1916) Clinical calorimetrytenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871. https://doi.org/10.1001/archinte.1916.00080130010002
Wu YL, Sequist LV, Tan EH et al (2018) Afatinib as first-line treatment of older patients with EGFR mutation-positive non-small-cell lung cancer: Subgroup analyses of the LUX-Lung 3, LUX-Lung 6, and LUX-Lung 7 trials. Clin Lung Cancer 19:e465–e479. https://doi.org/10.1016/j.cllc.2018.03.009
Ho GF, Chai CS, Alip A et al (2019) Real-world experience of first-line afatinib in patients with EGFR-mutant advanced NSCLC: a multicenter observational study. BMC Cancer 19:896. https://doi.org/10.1186/s12885-019-6107-1
Brueckl WM, Laack E, Reck M et al (2018) Efficacy of afatinib in the clinical practice: First results of the GIDEON trial: A prospective non-interventional study (NIS) in EGFR mutated NSCLC in Germany. Ann Oncol 28(8Suppl):viii393–viii547. https://doi.org/10.1093/annonc/mdy292
Kato T, Yoshioka H, Okamoto I, Yokoyama A, Hida T, Seto T, Kiura K, Massey D, Seki Y, Yamamoto N (2015) Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: Subgroup analysis of LUX-Lung 3. Cancer Sci 106:1202–1211. https://doi.org/10.1111/cas.12723
Liang SK, Hsieh MS, Lee MR, Keng LT, Ko JC, Shih JY (2017) Real-world experience of afatinib as a first-line therapy for advanced EGFR mutation-positive lung adenocarcinoma. Oncotarget 8:90430–90443. https://doi.org/10.18632/oncotarget.19563
Halmos B, Tan EH, Soo RA et al (2019) Impact of afatinib dose modification on safety and effectiveness in patients with EGFR mutation-positive advanced NSCLC: Results from a global real-world study (RealGiDo). Lung Cancer 127:103–111. https://doi.org/10.1016/j.lungcan.2018.10.028
Yang JC, Sequist LV, Zhou C et al (2016) Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol 27:2103–2110. https://doi.org/10.1093/annonc/mdw322
Yokoyama T, Yoshioka H, Fujimoto D et al (2019) A phase II study of low starting dose of afatinib as first-line treatment in patients with EGFR mutation-positive non-small-cell lung cancer (KTORG1402). Lung Cancer 135:175–180. https://doi.org/10.1016/j.lungcan.2019.03.030
Chae YK, Davis AA, Raparia K et al (2019) Association of Tumor Mutational Burden With DNA Repair Mutations and Response to Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer. Clin Lung Cancer 20:88–96
Nagahashi M, Sato S, Yuza K et al (2018) Common driver mutations and smoking history affect tumor mutation burden in lung adenocarcinoma. J Surg Res 230:181–185
Offin M, Rizvi H, Tenet M et al (2019) Tumor Mutation Burden and Efficacy of EGFR-Tyrosine Kinase Inhibitors in Patients with EGFR-Mutant Lung Cancers. Clin Cancer Res 25:1063–1069
Paz-Ares L, Tan EH, O’Byrne K et al (2017) Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 28:270–277. https://doi.org/10.1093/annonc/mdw611
Soria JC, Felip E, Cobo M et al (2015) Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 16:897–907. https://doi.org/10.1016/S1470-2045(15)00006-6
Srivastava RM, Trivedi S, Concha-Benavente F, Hyun-bae J, Wang L, Seethala RR, Branstetter BF 4th, Ferrone S, Ferris RL (2015) STAT1-induced HLA class i upregulation enhances immunogenicity and clinical response to anti-EGFR mAb cetuximab therapy in HNC patients. Cancer Immunol Res 3:936–945. https://doi.org/10.1158/2326-6066.CIR-15-0053
Pollack BP, Sapkota B, Cartee TV (2011) Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin Cancer Res 17:4400–4413. https://doi.org/10.1158/1078-0432.CCR-10-3283
Lizotte PH, Hong RL, Luster TA et al (2018) A high-throughput immune-oncology screen identifies EGFR inhibitors as potent enhancers of antigen-specific cytotoxic t-lymphocyte tumor cell killing. Cancer Immunol Res 6:1511–1523. https://doi.org/10.1158/2326-6066.CIR-18-0193
Schuler M, Wu YL, Hirsh V et al (2016) First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol 11:380–390. https://doi.org/10.1016/j.jtho.2015.11.014
Yang JC, Wu YL, Schuler M et al (2015) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16:141–151. https://doi.org/10.1016/S1470-2045(14)71173-8
Acknowledgements
We thank the staff members of the Department of Respiratory Medicine, Kitasato University School of Medicine for their assistance in data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Igawa, S., Ono, T., Kasajima, M. et al. Real-world assessment of afatinib for patients with EGFR-positive non-small cell lung cancer. Invest New Drugs 38, 1906–1914 (2020). https://doi.org/10.1007/s10637-020-00948-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00948-9