Abstract
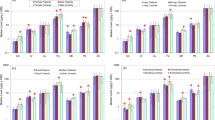
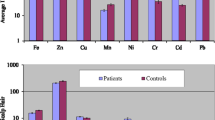
Thyroid cancer is among the most common type of head and neck cancer; diet, age, gender and environmental factors play vital roles in its malignancy. The present study was carried out to assess the imbalances in the contents of trace metals due to onset and progression of thyroid cancer. Scalp hair was used as matrix for the evaluation of toxic and trace metals. Quantification of the metals was done through atomic absorption spectrometry. In comparison with healthy subjects, the thyroid cancer patients revealed significantly higher median levels of Mn (71%), Co (64%), Cr (55%), K (49%), Fe (45%), Mg (42%), Pb (36%), Na (30%), and Ni (26%), while the median level of Zn was considerably lower in the patients. The correlation coefficients among the metals in the patients demonstrated significantly different communal relationships compared with the healthy counterparts. Multivariate methods exhibited noticeably dissimilar apportionment among the metals in the patients than the controls. Significant disparities in the metal levels were also noticed for various types (anaplastic thyroid cancer, follicular thyroid cancer, papillary thyroid cancer, and medullary thyroid cancer) as well as stages (I, II, III, and IV) among the thyroid cancer patients. Majority of the metals revealed perceptible disparities in their contents based on gender, habitat, dietary habits, and smoking habits of the patients and controls. Overall, the study showed significantly divergent distribution and associations of the essential and toxic metal levels in the scalp hair of the patients in comparison with the levels in controls.




Similar content being viewed by others
References
Zhao Z, Herman JG, Brock MV, Sheng J, Zhang M, Liu B, Guo M (2014) Methylation of DACT2 promotes papillary thyroid cancer metastasis by activating Wnt signaling. PloS One 9(11):e112336
Yang YJ, Na HJ, Suh MJ, Ban MJ, Byeon HK, Kim WS, Kim JW, Choi EC, Kwon HJ, Chang JW, Koh YW (2015) Hypoxia induces epithelial-mesenchymal transition in follicular thyroid cancer: involvement of regulation of twist by hypoxia inducible factor-1α. Yonsei Medical Journal 56(6):1503–1514
Carling T, Udelsman R (2014) Thyroid cancer. Annual Review of Medicine 65:125–137
Asa SL, Ezzat S (2018) The epigenetic landscape of differentiated thyroid cancer. Molecular and Cellular Endocrinology 469:3–10
La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E (2015) Thyroid cancer mortality and incidence: a global overview. International Journal of Cancer 136(9):2187–2195
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68(6):394–424
Wild C (2014) World cancer report (2014), C. P. Wild, & B. W. Stewart (Eds.). World Health Organization, Geneva, Switzerland
Noone AM, Cronin KA, Altekruse SF, Howlader N, Lewis DR, Petkov VI, Penberthy L (2017) Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992–2013. Cancer Epidemiology and Prevention Biomarkers 26(4):632–641
Guignard R, Truong T, Rougier Y, Baron-Dubourdieu D, Guénel P (2007) Alcohol drinking, tobacco smoking, and anthropometric characteristics as risk factors for thyroid cancer: a countrywide case-control study in New Caledonia. American Journal of Epidemiology 166(10):1140–1149
Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS (2009) Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiology and Prevention Biomarkers 18(3):784–791
Zhu X, Cheng SY (2017) Epigenetic modifications: novel therapeutic approach for thyroid cancer. Endocrinology and Metabolism 32(3):326–331
Jouybari L, Naz MS, Sanagoo A, Kiani F, Sayehmiri F, Sayehmiri K, Dehkordi AH (2018) Toxic elements as biomarkers for breast cancer: a meta-analysis study. Cancer Management and Research 10:69
Baltaci AK, Dundar TK, Aksoy F, Mogulkoc R (2017) Changes in the serum levels of trace elements before and after the operation in thyroid cancer patients. Biological Trace Element Research 175:57–64
Ismail PA, Yousif AM, Harki EM (2017) Alterations of some heavy metals and trace elements levels in breast cancer. Medicinal Chemistry 7:758–760
Farah IO, Nguyen PX, Arslan Z, Ayensu W, Cameron JA (2010) Significance of differential metal loads in normal versus cancerous cadaver tissues. Biomedical Sciences Instrumentation 46:404
Shan Z, Wei Z, Shaikh ZA (2018) Suppression of ferroportin expression by cadmium stimulates proliferation, EMT, and migration in triple-negative breast cancer cells. Toxicology and Applied Pharmacology 356:36–43
Janbabai G, Alipour A, Ehteshami S, Borhani SS, Farazmandfar T (2018) Investigation of trace elements in the hair and nail of patients with stomach cancer. Indian Journal of Clinical Biochemistry 33(4):450–455
Baltaci AK, Mogulkoc R, Baltaci SB (2019) The role of zinc in the endocrine system. Pakistan Journal of Pharmaceutical Sciences 32(1):231–239
Zhang T, Sun H, Kannan K (2013) Blood and urinary bisphenol A concentrations in children, adults, and pregnant women from China: partitioning between blood and urine and maternal and fetal cord blood. Environmental Science & Technology 47(9):4686–4694
Genuis SJ, Birkholz D, Rodushkin I, Beesoon S (2011) Blood, urine, and sweat (BUS) study: monitoring and elimination of bioaccumulated toxic elements. Archives of Environmental Contamination and Toxicology 61(2):344–357
Genuis SJ, Beesoon S, Birkholz D, Lobo RA (2012) Human excretion of bisphenol A: blood, urine, and sweat (BUS) study. Journal of Environmental and Public Health 2012:185731
Afridi HI, Kazi TG, Brabazon D, Naher S (2011) Association between essential trace and toxic elements in scalp hair samples of smokers rheumatoid arthritis subjects. Science of the Total Environment 412:93–100
Reiss B, Simpson CD, Baker MG, Stover B, Sheppard L, Seixas NS (2016) Hair manganese as an exposure biomarker among welders. Annals of Occupational Hygiene 60(2):139–149
Wołowiec P, Michalak I, Chojnacka K, Mikulewicz M (2013) Hair analysis in health assessment. Clinica Chimica Acta 419:139–171
Martín-Cameán A, Molina-Villalba I, Jos A, Iglesias-Linares A, Solano E, Cameán AM, Gil F (2014) Biomonitorization of chromium, copper, iron, manganese and nickel in scalp hair from orthodontic patients by atomic absorption spectrometry. Environmental Toxicology and Pharmacology 37:759–771
Nouioui MA, Araoud M, Milliand ML, Bessueille-Barbier F, Amira D, Ayouni-Derouiche L, Hedhili A (2018) Evaluation of the status and the relationship between essential and toxic elements in the hair of occupationally exposed workers. Environmental Monitoring and Assessment 190:731
Skröder H, Kippler M, Nermell B, Tofail F, Levi M, Rahman SM, Raqib R, Vahter M (2017) Major limitations in using element concentrations in hair as biomarkers of exposure to toxic and essential trace elements in children. Environmental Health Perspectives 125(6):067021
Jursa T, Stein CR, Smith DR (2018) Determinants of hair manganese, lead, cadmium and arsenic levels in environmentally exposed children. Toxics 6(2):19
Afzal A, Qayyum MA, Shah MH (2019) Study of trace metal imbalances in the scalp hair of stomach cancer patients with different types and stages. Biological Trace Element Research:1–10
Pasha Q, Malik SA, Shaheen N, Shah MH (2010) Investigation of trace metals in the blood plasma and scalp hair of gastrointestinal cancer patients in comparison with controls. Clinica Chimica Acta 411:531–539
Zhang LL, Lu L, Pan YJ, Ding CG, Xu DY, Huang CF, Pan XF, Zheng W (2015) Baseline blood levels of manganese, lead, cadmium, copper, and zinc in residents of Beijing suburb. Environmental Research 140:10–17
Cameron KS, Buchner V, Tchounwou PB (2011) Exploring the molecular mechanisms of nickel-induced genotoxicity and carcinogenicity: a literature review. Reviews on Environmental Health 26(2):81–92
Magaye R, Zhao J, Bowman L, Ding MI (2012) Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Experimental and Therapeutic Medicine 4(4):551–561
Thompson CM, Fedorov Y, Brown DD, Suh M, Proctor DM, Kuriakose L, Haws LC, Harris MA (2012) Assessment of Cr(VI)-induced cytotoxicity and genotoxicity using high content analysis. PloS One 7(8):e42720
Nickens KP, Patierno SR, Ceryak S (2010) Chromium genotoxicity: a double-edged sword. Chemico-Biological Interactions 188(2):276–288
Sun H, Brocato J, Costa M (2015) Oral chromium exposure and toxicity. Current Environmental Health Reports 2(3):295–303
Ludwig H, Evstatiev R, Kornek G, Aapro M, Bauernhofer T, Buxhofer-Ausch V, Fridrik M, Geissler D, Geissler K, Gisslinger H, Koller E (2015) Iron metabolism and iron supplementation in cancer patients. Wiener Klinische Wochenschrift 127(23-24):907–919
Jung M, Mertens C, Tomat E, Brüne B (2019) Iron as a central player and promising target in cancer progression. International Journal of Molecular Sciences 20(2):273
Pfeifhofer-Obermair C, Tymoszuk P, Petzer V, Weiss G, Nairz M (2018) Iron in the tumor microenvironment—connecting the dots. Frontiers in Oncology 8:549
de Morais RM, Sobrinho AB, de Souza Silva CM, de Oliveira JR, da Silva IC, de Toledo NO (2018) The role of the NIS (SLC5A5) gene in papillary thyroid cancer: a systematic review. International Journal of Endocrinology 2018:9128754
Denoyer D, Masaldan S, La Fontaine S, Cater MA (2015) Targeting copper in cancer therapy: ‘copper that cancer’. Metallomics 7(11):1459–1476
Kalinowski DS, Stefani C, Toyokuni S, Ganz T, Anderson GJ, Subramaniam NV, Trinder D, Olynyk JK, Chua A, Jansson PJ, Sahni S (2016) Redox cycling metals: pedaling their roles in metabolism and their use in the development of novel therapeutics. Biochimica et Biophysica Acta 1863(4):727–748
Ostadrahimi A, Payahoo L, Somi MH, Khajebishak Y (2017) The association between urinary cadmium levels and dietary habits with risk of gastrointestinal cancer in Tabriz, Northwest of Iran. Biological Trace Element Research 175(1):72–78
Skalny AV, Skalnaya MG, Grabeklis AR, Zhegalova IV, Serebryansky EP, Demidov VA, Salnikova EV, Uzhentseva MS, Lobanova YN, Skalny AA, Tinkov AA (2018) Interactive effects of age and gender on levels of toxic and potentially toxic metals in children hair in different urban environments. International Journal of Environmental Analytical Chemistry 98(6):520–535
Malandrino P, Russo M, Ronchi A, Minoia C, Cataldo D, Regalbuto C, Giordano C, Attard M, Squatrito S, Trimarchi F, Vigneri R (2016) Increased thyroid cancer incidence in a basaltic volcanic area is associated with non-anthropogenic pollution and biocontamination. Endocrine 53(2):471–479
Peterson E, De P, Nuttall R (2012) BMI, diet and female reproductive factors as risks for thyroid cancer: a systematic review. PloS One 7(1):e29177
Wiersinga WM (2013) Smoking and thyroid. Clinical Endocrinology 79(2):145–151
Pasupathi P, Pichandi S (2011) Chronic tobacco smoking and gastric cancer: a review. International Journal of Current Biomedical and Pharmaceutical Research 1(2):48–66
Fasinu PS, Orisakwe OE (2013) Heavy metal pollution in sub-Saharan Africa and possible implications in cancer epidemiology. Asian Pacific Journal of Cancer Prevention 14(6):3393–3402
Wei B, Yang L, Yu J, Ye B, Jia X (2013) Are metals accumulated in human hair affected by naturally occurring asbestos fiber contamination? A case study from a rural area of China. Biological Trace Element Research. 156(1-3):12–21
Acknowledgments
We are grateful to the administrations of the Nuclear Oncology and Radiotherapy Institute (NORI), Islamabad, Pakistan, for their invaluable help during the sample collection. Technical and financial help by the Quaid-i-Azam University, Islamabad, Pakistan, to execute this project is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Ethical Review Committee, NORI, Islamabad, Ref. No. QAUC-2017-A77) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 158 kb)
Rights and permissions
About this article
Cite this article
Bibi, K., Shah, M.H. Study of Essential and Toxic Metal Imbalances in the Scalp Hair of Thyroid Cancer Patients in Comparison with Healthy Donors. Biol Trace Elem Res 199, 500–512 (2021). https://doi.org/10.1007/s12011-020-02186-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02186-9