Abstract
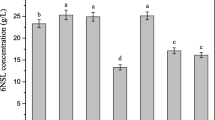
The major troubles in 6-(N-hydroxyethyl)-amino-6-deoxy-α-l-sorbofuranose (6NSL) production from N-2-hydroxyethyl glucamine (NHEG) by Gluconobacter oxydans were low cell yield during cell preparation and loss of cells’ biocatalytic ability during biotransformation, resulting in high production cost and low 6NSL production. The target of this work was to enhance 6NSL production by reusing cells and improving the cells biocatalytic ability. First, inhibitory effects of substrate and product on 6NSL production, and optimization of cell regeneration condition were investigated, respectively. Then repeated production of 6NSL by immobilized cell using a strategy of in situ exhaustive cell regeneration in a bubble column bioreactor was developed. As a result, the bioprocess underwent nine cycles, the average 6NSL production and conversion rate of NHEG to 6NSL reached 42.6 g L−1 and 83.1% in each batch was achieved, respectively.






Similar content being viewed by others
References
Schedel M (2008) Regioselective oxidation of aminosorbitol with Gluconobacter oxydans, key reaction in the industrial 1-deoxynojirimycin synthesis. In: Rehm H-J, Reed G (eds) Biotechnology: Biotransformations II, vol 8. Wiley, New Jersey
Scott LJ, Spencer CM (2000) Miglitol: a review of its therapeutic potential in type 2 diabetes mellitus. Drugs 59:521–549
Grabner Roy W, Landis Bryan H, Wang Ping TU, Prunier Michael LEE, Scaros Mike G (1999) Process for microbially oxidizing N-substituted glucamines. US Patent 0019837
Gupta A, Singh VK, Qazi GN, Kumar A (2001) Gluconobacter oxydans: Its biotechnological applications. J Mol Microbiol Biotechnol 3:445–456
Hanke T (2010) Studies on central carbon metabolism and respiration of Gluconobacter oxydans 621H. PhD Thesis, Düsseldorf University, Düsseldorf, Germany
Bringer S, Bott M (2016) Central carbon metabolism and respiration in Gluconobacter oxydans. In: Matsushita K, Toyama H, Tonouchi N, Okamoto-Kainuma A (eds) Acetic acid bacteria: ecology and physiology. Springer Japan, Tokyo
Du J, Bai W, Song H, Yuan YJ (2013) Combinational expression of sorbose/sorbosone dehydrogenases and cofactor pyrroloquinoline quinone increases 2-keto-L-gulonic acid production in Ketogulonigenium vulgare-Bacillus cereus consortium. Metab Eng 19:50–56
Hu ZC, Bu JL, Wang RY, Ke X, Zheng YG (2018) Enhanced production of 6-(N-Hydroxyethyl)-amino-6-deoxy-α-L-sorbofuranose by immobilized Gluconobacter oxydanson corn stover with a pH control strategy in a bubble column bioreactor. Appl Biochem Biotechnol 188:297–309
Ke X, Yu PH, Hu ZC, Chen L, Sun XQ, Zheng YG (2019) Synergistic improvement of PQQ-dependent D-sorbitol dehydrogenase activity from Gluconobacter oxydans for the biosynthesis of miglitol precursor 6-(N-hydroxyethyl)-amino-6-deoxy-α-L-sorbofuranose. J Biotechnol 300:55–62
Dikshit PK, Moholkar VS (2016) Kinetic analysis of dihydroxyacetone production from crude glycerol by immobilized cells of Gluconobacter oxydans MTCC 904. Bioresour Technol 216:948–957
Dikshit PK, Padhi SK, Moholkar VS (2017) Process optimization and analysis of product inhibition kinetics of crude glycerol fermentation for 1,3-Dihydroxyacetone production. Bioresour Technol 244:362–370
Hekmat D, Bauer R, Neff V (2007) Optimization of the microbial synthesis of dihydroxyacetone in a semi-continuous repeated-fed-batch process by in situ immobilization of Gluconobacter oxydans. Process Biochem 42:71–76
Richhardt J, Bringer S, Bott M (2012) Mutational analysis of the pentose phosphate and Entner-Doudoroff pathways in Gluconobacter oxydans reveals improved growth of a delta edd delta eda mutant on mannitol. Appl Environ Microbiol 78:6975–6986
Kiefler I, Bringer S, Bott M (2017) Metabolic engineering of Gluconobacter oxydans 621H for increased biomass yield. Appl Microbiol Biotechnol 101:5453–5467
Prust C, Hoffmeister M, Liesegang H, Wiezer A, Fricke WF, Ehrenreich A, Gottschalk G, Deppenmeier U (2005) Complete genome sequence of the acetic acid bacterium Gluconobacter oxydans. Nat Biotechnol 23:195–200
Ke X, Wang NN, Yu PH, Lu YH, Hu ZC, Zheng YG (2018) Biosynthesis of miglitol intermediate 6-(N-hydroxyethyl)-amino-6-deoxy-α-l-sorbofuranose by an improved d-sorbitol dehydrogenase from Gluconobacter oxydans. 3 Biotech 8:231
Wei SH, Song QX, Wei DZ (2007) Repeated use of immobilized Gluconobacter oxydans cells for conversion of glycerol to dihydroxyacetone. Prep Biochem Biotechnol 37:67–76
Hu ZC, Tian SY, Ruan LJ, Zheng YG (2017) Repeated biotransformation of glycerol to 1,3-dihydroxyacetone by immobilized cells of Gluconobacter oxydans with glycerol- and urea-feeding strategy in a bubble column bioreactor. Bioresour Technol 233:144–149
Hua X, Zhou X, Xu Y (2018) Improving techno-economics of bioproduct glycolic acid by successive recycled-cell catalysis of ethylene glycol with Gluconobacter oxydans. Bioprocess Biosyst Eng 41:1555–1559
Zhang Y, Gao F, Zhang SP, Su ZG, Ma GH, Wang P (2011) Simultaneous production of 1,3-dihydroxyacetone and xylitol from glycerol and xylose using a nanoparticle-supported multi-enzyme system with in situ cofactor regeneration. Bioresour Technol 102:1837–1843
Li MH, Wu J, Liu X, Lin JP, Wei DZ, Chen H (2010) Enhanced production of dihydroxyacetone from glycerol by overexpression of glycerol dehydrogenase in an alcohol dehydrogenase-deficient mutant of Gluconobacter oxydans. Bioresour Technol 101:8294–8299
Claret C, Bories A, Soucaille P (1993) Inhibitory effect of dihydroxyacetone on Gluconobacter oxydans: kinetic aspects and expression by mathematical equations. J Ind Microbiol 11:105–112
Luong JH (1985) Kinetics of ethanol inhibition in alcohol fermentation. Biotechnol Bioeng 27:280–285
Yang XP, Zhong GF, Lin JP, Mao DB, Wei DZ (2010) Pyrroloquinoline quinone biosynthesis in Escherichia coli through expression of the Gluconobacter oxydans pqqABCDE gene cluster. J Ind Microbiol Biotechnol 37:575–580
Tkac J, Svitel J, Vostiar I, Navratil M, Gemeiner P (2009) Membrane-bound dehydrogenases from Gluconobacter sp.: Interfacial electrochemistry and direct bioelectrocatalysis. Bioelectrochem 76:53–62
Dikshit PK, Moholkar VS (2019) Batch and repeated-batch fermentation for 1,3-dihydroxyacetone production from waste glycerol using free, immobilized and resting Gluconobacter oxydans cells. Waste Biomass Valori 10:2455–2465
Duine JA, Jongejan JA (1989) Quinoproteins, enzymes with pyrrolo-quinoline quinone as cofactor. Annu Rev Biochem 58:403–426
Matsushita K, Arents JC, Bader R, Yamada M, Adachi O, Postma PW (1997) Escherichia coli is unable to produce pyrroloquinoline quinone (PQQ). Microbiol 143:3149–3156
Sheng BB, Xu J, Zhang YX, Jiang TY, Deng SS, Kong J, Gao C, Ma CQ, Xu P (2015) Utilization of D-Lactate as an energy source supports the growth of Gluconobacter oxydans. Appl Environ Microbiol 81:4098–4110
Su WW, Wu XG, Chen DJ, Xia HZ (2007) Biotransformation of 6-(N-hydroxyethyl)amino-6-deoxy-L-sorbofuranose by immobilized Gluconobacter oxydans. J Shenyang Pharm Univ 2:118–122
Zhang JB, Zhang XL, Wang DH, Zhao BX, He G (2011) Biocatalytic Regioselective Oxidation of N-hydroxyethyl glucamine for Synthesis of Miglitol. Adv Mater Res 197–198:51–55
Ke X, Lu YH, Yu PH, Hu ZC, Chen L, Sun XQ, Zheng YG (2019) Glutamate addition improves the activity of membrane-bound sorbitol dehydrogenase in a pyrroloquinoline quinone-dependent manner: A feasible strategy for the cost-effective fermentation of Gluconobacter oxydans. Process Biochem 84:1–8
Acknowledgements
This study was funded by National Major Project of Scientific Instruments Development of China (2012YQ15008713), granted by the Ministry of Science and Technology of PR China.
Author information
Authors and Affiliations
Contributions
HZ and ZY designed this study; HZ and ZZ conducted the experiments; HZ, ZZ, and KX analyzed the data; HZ and ZZ wrote the paper; KX revised the manuscript. All the authors have read the manuscript critically.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there is no conflict of interest.
Ethical statement
The authors declare that there are no studies conducted with human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, ZC., Zhao, ZY., Ke, X. et al. Repeated production of 6-(N-hydroxyethyl)-amino-6-deoxy-α-l-sorbofuranose by immobilized Gluconobacter oxydans cells with a strategy of in situ exhaustive cell regeneration. Bioprocess Biosyst Eng 43, 1781–1789 (2020). https://doi.org/10.1007/s00449-020-02368-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02368-8