Abstract
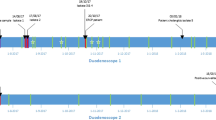
Endoscope contamination is infrequent but can be the source of nosocomial infections and outbreaks. In August 2016, an unexpected increase in the incidence of amikacin-resistant P. aeruginosa isolates (AK-Pae) was observed at a tertiary care center in the south of Spain. An epidemiological and microbiological investigation (August-October 2016) was performed to explain this finding. Isolates from clinical and environmental samples (2 endoscopes used for retrograde cholangiopancreatography; ERCP) were identified by MALDI-TOF. Antimicrobial susceptibility testing was performed using the MicroScan system. Whole-Genome-Sequencing (Miseq, Illumina) was performed to determine the resistome and virulome. Clonal relatedness among isolates was assessed by SpeI-PFGE and MLST. A Caenorhabditis elegans killing assay was performed for virulence testing. Biofilm formation was performed using a colorimetric assay. Four of the 5 patients infected and/or colonized with AK-Pae in August 2016 had undergone ERCP ≤5 days before sample collection. Two endoscopes were contaminated with AK-Pae. Isolates from one endoscope showed an identical PFGE pattern to 9 isolates (cluster I) and differed (1–2 bands) to 5 isolates (cluster II). Isolates from these clusters belonged to the ST17 clone. This S17 clone was characterized by its low virulence in the C. elegans killing assay, and its biofilm-forming ability, slightly superior to that of high-risk clones of P. aeruginosa ST175 and ST235. This outbreak was caused by an endoscope used for ERCP contaminated with an invasive, moderately virulent, biofilm-forming AK-Pae ST17 clone, suggesting the possible emergence of a new high-risk lineage of this clone.




Similar content being viewed by others
References
Valentini M, Gonzalez D, Mavridou DA, Filloux A (2018) Lifestyle transitions and adaptive pathogenesis of Pseudomonas aeruginosa. Curr Opin Microbiol 41:15–20
Oliver A, Mulet X, López-Causapé C, Juan C (2015) The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist Updat 21-22:41–59
Ciofu O, Tolker-Nielsen T (2019) Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents-how P. aeruginosa can escape antibiotics. Front Microbiol 10:913
Hervé RC (2017) Endoscopy in the twenty-first century: minimally invasive state-of-the-art medical technology or a future main vector of hospital-acquired infections? J Hosp Infect 97:329–330
Rahman MR, Perisetti A, Coman R, Bansal P, Chhabra R, Goyal H (2019) Duodenoscope-associated infections: update on an emerging problem. Dig Dis Sci 64:1409–1418
Saliou P, Baron R (2015) Outbreaks linked to duodenoscopes: microbiological control should be improved. Endoscopy 47:1058
Beilenhoff U, Biering H, Blum R, Brljak J, Cimbro M, Dumonceau JM et al (2018) Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) – update 2018. Endoscopy 50:1205–1234
García-Castillo M, Del Campo R, Morosini MI, Riera E, Cabot G, Willems R et al (2011) Wide dispersion of ST175 clone despite high genetic diversity of carbapenem-nonsusceptible Pseudomonas aeruginosa clinical strains in 16 Spanish hospitals. J Clin Microbiol 49:2905–2910
García I, Ballesta S, Gilaberte Y, Rezusta A, Pascual Á (2015) Antimicrobial photodynamic activity of hypericin against methicillin-susceptible and resistant Staphylococcus aureus biofilms. Future Microbiol 10:347–356
Sánchez-Diener I, Zamorano L, López-Causapé C, Cabot G, Mulet X, Peña C et al (2017) Interplay among resistance profiles, high-risk clones, and virulence in the Caenorhabditis elegans Pseudomonas aeruginosa infection model. Antimicrob Agents Chemother 61:e01586–e01517
Fraser TG, Reiner S, Malczynski M, Yarnold PR, Warren J, Noskin GA (2004) Multidrug-resistant Pseudomonas aeruginosa cholangitis after endoscopic retrograde cholangiopancreatography: failure of routine endoscope cultures to prevent an outbreak. Infect Control Hosp Epidemiol 25:856–859
Robertson P, Smith A, Mead A, Smith I, Khanna N, Wright P et al (2015) Risk-assessment-based approach to patients exposed to endoscopes contaminated with Pseudomonas spp. J Hosp Infect 90:66–69
Kizny Gordon AE, Mathers AJ, Cheong EYL, Gottlieb T, Kotay S, Walker AS et al (2017) The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections-a systematic review of the literature. Clin Infect Dis 64:1435–1444
Decraene V, Ghebrehewet S, Dardamissis E, Huyton R, Mortimer K, Wilkinson D et al (2018) An outbreak of multidrug-resistant Pseudomonas aeruginosa in a burns service in the north of England: challenges of infection prevention and control in a complex setting. J Hosp Infect 100:e239–e245
Gutiérrez O, Juan C, Cercenado E, Navarro F, Bouza E, Coll P et al (2007) Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa isolates from Spanish hospitals. Antimicrob Agents Chemother 51:4329–4335
Quale J, Bratu S, Gupta J, Landman D (2006) Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother 50:1633–1641
Danel F, Hall LM, Gur D, Livermore DM (1995) OXA-14, another extended-spectrum variant of OXA-10 (PSE-2) beta-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother 39:1881–1884
Alfa MJ (2019) Biofilms on instruments and environmental surfaces: do they interfere with instrument reprocessing and surface disinfection? Review of the literature. Am J Infect Control 47S:A39–A45
Lee JY, Peck KR, Ko KS (2013) Selective advantages of two major clones of carbapenem-resistant Pseudomonas aeruginosa isolates (CC235 and CC641) from Korea: antimicrobial resistance, virulence and biofilm-forming activity. J Med Microbiol 62:1015–1024
Römling U, Kader A, Sriramulu DD, Simm R, Kronvall G (2005) Worldwide distribution of Pseudomonas aeruginosa clone C strains in the aquatic environment and cystic fibrosis patients. Environ Microbiol 7:1029–1038
Mustafa MH, Chalhoub H, Denis O, Deplano A, Vergison A, Rodriguez-Villalobos H et al (2016) Antimicrobial susceptibility of Pseudomonas aeruginosa isolated from cystic fibrosis patients in northern Europe. Antimicrob Agents Chemother 60:6735–6741
Acknowledgments
We would like to thank the Reference Laboratory, Program for the Prevention and Control of Healthcare-Associated Infections and Antimicrobial Stewardship in Andalucía (PIRASOA, Servicio Andaluz de Salud) for their collaboration.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernández-Cuenca, F., López-Cerero, L., Cabot, G. et al. Nosocomial outbreak linked to a flexible gastrointestinal endoscope contaminated with an amikacin-resistant ST17 clone of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis 39, 1837–1844 (2020). https://doi.org/10.1007/s10096-020-03915-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-020-03915-7