Abstract
Background
Although pure cerebellar ataxia is usually emphasized as the characteristic clinical feature of spinocerebellar ataxia type 6 (SCA6), parkinsonism has been repeatedly described in patients with genetically confirmed SCA6.
Methods
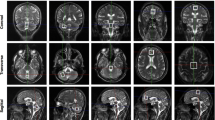
We conducted a positron emission tomography study using a combination of [18F]fluoro-l-dopa for dopamine synthesis and [11C]raclopride for dopamine D2 receptor function on six genetically confirmed SCA6 patients, both with and without parkinsonism. To the best of our knowledge, this is the first dopamine receptor imaging study of patients with SCA6.
Results
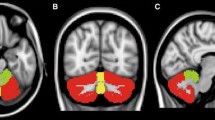
Most patients had somewhat decreased dopaminergic function, and this decrease was significant in the caudate nucleus. In addition, one SCA6 patient with parkinsonism had whole striatal dysfunction of both dopamine synthesis and dopamine D2 receptor function.
Conclusions
The pathology of SCA6 may not be restricted to the cerebellum, but may also be distributed across various regions, including in both presynaptic and postsynaptic dopaminergic neurons to some degree. Patients with SCA6 may show apparent parkinsonism after the progression of neurodegeneration.

Similar content being viewed by others
References
Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC (1997) Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet 15:62–69
Geschwind DH, Perlman S, Figueroa KP, Karrim J, Baloh RW, Pulst SM (1997) Spinocerebellar ataxia type 6: frequency of the mutation and genotype-phenotype correlations. Neurology 49:1247–1251
Gomez CM, Thompson RM, Gammack JT, Perlman SL, Dobyns WB, Truwit CL, Zee DS, Clark HB, Anderson JH (1997) Spinocerebellar ataxia type 6: gaze evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann Neurol 42:933–950
Takahashi H, Ikeuchi T, Honma Y, Hayashi S, Tsuji S (1998) Autosomal dominant cerebellar ataxia (SCA6): clinical, genetic and neuropathological study in a family. Acta Neuropathol 95:333–337
Schöls L, Krüger R, Amoiridis G, Przuntek H, Epplen JT, Riess O (1998) Spinocerebellar ataxia type 6: genotype and phenotype in German kindreds. J Neurol Neurosurg Psychiatry 64:67–73
Lee WY, Jin DK, Oh MR, Lee JE, Song SM, Lee EA, Kim GM, Chung JS, Lee KH (2003) Frequency analysis and clinical characterization of spinocerebellar ataxia types 1, 2, 3, 6, and 7 in Korean patients. Arch Neurol 60:858–863
Khan NL, Giunti P, Sweeney MG, Scherfler C, Brien MO, Piccini P, Wood NW, Lees AJ (2005) Parkinsonism and nigrostriatal dysfunction are associated with spinocerebellar ataxia type 6 (SCA6). Mov Disord 20:1115–1119
Schmitz-Hübsch T, Coudert M, Bauer P, Giunti P, Globas C, Baliko L, Filla A, Mariotti C, Rakowicz M, Charles P, Ribai P, Szymanski S, Infante J, van de Warrenburg BP, Dürr A, Timmann D, Boesch S, Fancellu R, Rola R, Depondt C, Schöls L, Zdienicka E, Kang JS, Döhlinger S, Kremer B, Stephenson DA, Melegh B, Pandolfo M, di Donato S, du Montcel ST, Klockgether T (2008) Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms. Neurology 71:982–989
Kim JM, Lee JY, Kim HJ, Kim JS, Kim YK, Park SS, Kim SE, Jeon BS (2010) The wide clinical spectrum and nigrostriatal dopaminergic damage in spinocerebellar ataxia type 6. J Neurol Neurosurg Psychiatry 81:529–532
Soong B, Liu R, Wu L, Lu Y, Lee H (2001) Metabolic characterization of spinocerebellar ataxia type 6. Arch Neurol 58:300–304
Garnett ES, Firnau G, Nahmias C (1983) Dopamine visualized in the basal ganglia of living man. Nature 305:137–138
Farde L, Ehrin E, Eriksson L, Greitz T, Hall H, Hedström CG, Litton JE, Sedvall G (1985) Substituted benzamides as ligands for visualization of dopamine receptor binding in the human brain by positron emission tomography. Proc Natl Acad Sci USA 82:3863–3867
Dhawan V, Ma Y, Pillai V, Spetsieris P, Chaly T, Belakhlef A, Margouleff C, Eidelberg D (2002) Comparative analysis of striatal FDOPA uptake in Parkinson’s disease: ratio method versus graphical approach. J Nucl Med 43:1324–1330
Farde L, Hall H, Ehrin E, Sedvall G (1986) Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 231:258–261
Lidow MS, Goldman-Rakic PS, Rakic P, Innis RB (1989) Dopamine D2 receptors in the cerebral cortex: Distribution and pharmacological characterization with [3Hlraclopride. Proc Natl Acad Sci USA 86:6412–6416
Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schöls L, Szymanski S, van de Warrenburg BP, Dürr A, Klockgether T, Fancellu R (2006) Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66:1717–1720
Yun JY, Kim JM, Kim HJ, Kim YE, Jeon BS (2012) SCA6 presenting with young-onset parkinsonism without ataxia. Mov Disord 27:1067–1068
Takeshima S, Takeda I, Kobatake K, Yamashita T, Abe K, Kuriyama M (2015) SCA6 presenting parkinsonism without ataxia—a case report. Rinsho Shinkeigaku 55:243–247
Wüllner U, Reimold M, Abele M, Bürk K, Minnerop M, Dohmen BM, Machulla HJ, Bares R, Klockgether T (2005) Dopamine transporter positron emission tomography in spinocerebellar ataxias type 1, 2, 3, and 6. Arch Neurol 62:1280–1285
Xie T, Appelbaum D, Bernard J, Padmanaban M, Pu Y, Gomez C (2016) Evaluation of parkinsonism and striatal dopamine transporter loss in patients with spinocerebellar ataxia type 6. J Neurol 263:2302–2307
Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, Bannister R, Marsden CD, Frackowiak RS (1990) Differing patterns of striatal 18F-dopa uptake in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol 28:547–555
Graybiel AM, Hirsch EC, Agid Y (1990) The nigrostriatal system in Parkinson's disease. Adv Neurol 53:17–29
Reetz K, Costa AS, Mirzazade S, Lehmann A, Juzek A, Rakowicz M, Boguslawska R, Schöls L, Linnemann C, Mariotti C, Grisoli M, Dürr A, van de Warrenburg BP, Timmann D, Pandolfo M, Bauer P, Jacobi H, Hauser TK, Klockgether T, Schulz JB, the Ataxia Study Group Investigators (2013) Genotype-specific patterns of atrophy progression are more sensitive than clinical decline in SCA1, SCA3 and SCA6. Brain 136(Pt 3):905–917
Fearnley JM, Lees AJ (1991) Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 114(Pt 5):2283–2301
Greffard S, Verny M, Bonnet AM, Beinis JY, Gallinari C, Piette F, Meaum S, Hauw JJ, Duyckaerts C (2006) Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol 63:584–588
Hilker R, Schweitzer K, Coburger S, Ghaemi M, Weisenbach S, Jacobs AH, Rudolf J, Herholz K, Heiss WD (2005) Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F18 activity. Arch Neurol 62:378–382
Acknowledgements
This study was funded by The Rehabilitation Research Fund of Social Welfare Corporation Nagoya City Rehabilitation Agency. We are especially grateful to the late Dr. Michitaka Matsubara, former Director of Department of Planning and Research, Nagoya City Rehabilitation Center, and to the late Professor Tomoyuki Shirai, former President of Nagoya City Rehabilitation Center and Professor Emeritus of Nagoya City University, for their kind suggestions during their lifetimes. We also thank Mr. Shin Hibino, Department of Planning and Research, Nagoya City Rehabilitation Center, for his excellent technical assistance.
Funding
This study was funded by The Rehabilitation Research Fund of Social Welfare Corporation Nagoya City Rehabilitation Agency.
Author information
Authors and Affiliations
Contributions
All of the authors have read the manuscript and have approved this submission. YH: Recruitment of patients. Assessment of parkinsonism. EH: Scanning and analyzing of images. YI: Scanning of images. AI: Scanning and analyzing of images. YG: Recruitment of patients. Assessment of parkinsonism. SK: Recruitment of patients. Assessment of parkinsonism. KO: Recruitment of patients. Assessment of parkinsonism. TK: Recruitment of patients. Assessment of parkinsonism. CS: Recruitment of patients. TT: Recruitment of patients. AI: Recruitment of patients. KN: Recruitment of patients. HH: Recruitment of patients. NM: Recruitment of patients. KY: Recruitment of patients. HK: Recruitment of patients.
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest or competing interests to declare.
Ethics approval
This study was approved by The Ethics Committee of Nagoya City Rehabilitation Center.
Informed Consent
All participants gave written informed consent to participate.
Consent for publication
All authors have read and agree with the contents of the manuscript for publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Horimoto, Y., Hayashi, E., Ito, Y. et al. Dopaminergic function in spinocerebellar ataxia type 6 patients with and without parkinsonism. J Neurol 267, 2692–2696 (2020). https://doi.org/10.1007/s00415-020-09908-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09908-y