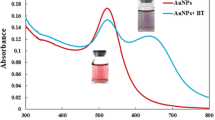
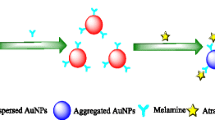
An innovative approach was developed to determine benzotriazole (BTA) in aqueous solutions. This method was based on surface plasmon resonance (SPR) property of gold nanoparticles (AuNPs). The reaction between gold nanoparticles and benzotriazole occurred. Then, benzotriazole was determined by spectrophotometry. Also, transmission electron microscopy (TEM) was used to show aggregation of gold nanoparticles in the presence of BTA. The effect of various parameters such as pH, contact time, concentration of gold nanoparticles, amount of buffer, and different surfactant was investigated. The proposed method is capable of determining BTA in the range of 10–100 μg/L with a limit of detection (LOD) 5 μg/L and limit of quantifi cation (LOQ) 16 μg/L. In addition, the relative standard deviation (RSD) of this method was 2.5 and 1%. Also, benzotriazole was measured in real water samples.
Similar content being viewed by others
References
E. Jover, V. Matamoros, and J. Maria Bayona, J. Chromatogr. A, 1216, 4013–4019 (2009).
Y. S. Liu, G.G. Ying, A. Shareef, and R. S. Kookan, Environ. Pollut., 165, 225–232 (2012).
Y. Li, J. Wang, W. Guo, C. Gao, and Z. Cheng, Instrum. Sci. Technol., 45, 290–300 (2017).
L. Hamenu, A. Madzvamuse, and L. Mohammed, J. Ind. Eng. Chem., 53, 241–246 (2017).
M. Lv, J. Ma, Q. Li, and Hui Xu, Bioorg. Med. Chem. Lett., 28, 181–187 (2018).
G. D. Breedveld, R. Roseth, M. Sparrevik, T. Hartnlk, and L. J. Hem, Water, Air, Soil Pollut., 3, 91–101 (2003).
G. K. Patil, H. C. Patil, I. M. Patil, S. L. Borse, and S. P. Pawar, World J. Pharm. Pharm. Sci., 4, 532–548 (2015).
N. P. Milosevic, V. B. Dimova, and N. U. Perisic-Janjic, Eur. J. Pharm. Sci., 49, 10–17 (2013).
M. M. Mennucci, E. P. Banczek, P. R. P. Rodrigues, and I. Costa, Cement Concrete Compos., 31, 418–424 (2009).
K. Wang, H. W. Pickering, and K. G. Weil, J. Electrochem. Soc., 150, B176–B180 (2003).
Z. Zhang, N. Ren, Y.F. Li, T. Kunisue, D. Gao, and K. Kannan, Environ. Sci. Technol., 45, 3909–3916 (2011).
W. Giger, C. Schaffner, and H. P. Kohler, Environ. Sci. Technol., 40, 7186–7192 (2006).
R. Loos, G. Locoro, S. Comero, S. Contini, D. Schwesig, F. Werres, P. Balsaa, O. Gans, S. Weiss, L. Blaha, M. Bolchi, and B. Manfred Gawlik, Water Res., 44, 4115–4126 (2010).
C. Dominguez, C. Reyes-Contreras, and J. M. Bayona, J. Chromatogr. A, 1230, 117–122 (2012).
N. Haji Seyed Javadi, M. Baghdadi, N. Mehrdadi, and M. Mortazavi, J. Environ. Chem. Eng., 6, 6421–6430 (2018).
P. Herrero, F. Borrull, R. M. Marce, and E. Pocurull, J. Chromatogr. A, 1355, 53–60 (2014).
A. Naccarato, E. Gionfriddo, G. Sindona, and A. Tagarelli, J. Chromatogr. A, 1338, 164–173 (2014).
E. Patsalides and K. Robards, J. Chromatogr., 331, 149–160 (1985).
W. Xu, W. Yan, and T. Licha, J. Chromatogr. A, 1422, 270–276 (2015).
J. Casado, I. Rodriguez, I. Carpinteiro, M. Ramil, and R. Cela, J. Chromatogr. A, 1293, 126–132 (2013).
Y. S. Liu, G. G. Ying, A. Shareef, and R. S. Kookana, J. Chromatogr. A, 1218, 5328–5335 (2011).
L. Jing, W. Meng-Meng, W. Qiang, L. Hai-Pu, and Y. Zhao-Guang, Chin. J. Anal. Chem., 46, 1817–1824 (2018).
R. Asrariyan and S. Elhami, Chem. Pap., 71, 2301–2308 (2017).
Y. Tang and X. Zeng, J. Chem. Ed., 87, 742–746 (2010).
H. Parham, N. Pourreza, and F. Marahel, Spectrochim. Acta, Mol. Biomol. Spectrosc., 151, 308–314 (2015).
J. N. Miller and J. C Miller, Statistics and Chemometrics for Analytical Chemistry, 6th ed., ISBN-978-0-273-73042-2 (2010).
A. A. Szalay, P. J. Hill, and L. J. Kricka, Bioluminescence and Chemiluminescence: Chemistry, Biology and Applications, 1st ed., World Scientifi c Publ. (2007).
P. Herrero, F. Borrull, E. Pocurull, and R. M. Marce, J. Chromatogr. A, 1309 (2013) 22–32.
A. Speltini, M. Sturini, F. Maraschi, A. Porta, and A. Profumo, Talanta, 147 (2016) 322–327.
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 87, No. 2, p. 347, March–April, 2020.
Rights and permissions
About this article
Cite this article
Esmaile, N., Sohrabi, M.R. & Motiee, F. Rapid and Sensitive Spectrophotometry Method Based on Gold Nanoparticles for Trace Determination of Benzotriazole in Aqueous Solutions. J Appl Spectrosc 87, 372–377 (2020). https://doi.org/10.1007/s10812-020-01009-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-020-01009-y