Transcriptomic Evidence of Molecular Mechanisms Underlying the Response of Lactobacillus plantarum WCFS1 to Hydroxytyrosol
Abstract
:1. Introduction
2. Material and Methods
2.1. Bacterial Strain, Culture Conditions
2.2. RNA Extraction
2.3. Microarray: cDNA Synthesis, Purification and Hybridization
2.4. Data Analysis
2.5. Microarray Data Accession Number
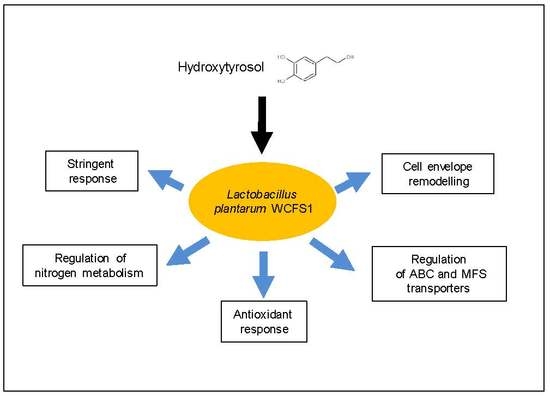
3. Results and Discussion
3.1. Global Analysis of the Lactobacillus Plantarum wcfs1 Transcriptomic Response to Hydroxytyrosol
3.2. Antioxidant Responses Triggered by Hydroxytyrosol
3.3. RNA Degradation
3.4. Elements Typically Involved in the Stringent Response Are Responsive to HXT
3.5. Cell Envelope Modifications in Response to HXT Stress
3.6. Energy Metabolism
3.7. Nitrogen Metabolism
3.8. Regulation of ABC-Transporters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bayram, B.; Esatbeyoglu, T.; Schulze, N.; Özçelik, B.; Frank, J.; Rimbach, G. Comprehensive Analysis of Polyphenols in 55 Extra Virgin Olive Oils by HPLC-ECD and Their Correlation with Antioxidant Activities. Plant Foods Hum. Nutr. 2012, 67, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2019, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curiel, J.A.; Rodríguez, H.; Rivas, B.D.L.; Anglade, P.; Baraige, F.; Zagorec, M.; Champomier-Vergès, M.; Muñoz, R.; De Felipe, F.L. Response of a Lactobacillus plantarum human isolate to tannic acid challenge assessed by proteomic analyses. Mol. Nutr. Food Res. 2011, 55, 1454–1465. [Google Scholar] [CrossRef]
- Reverón, I.; Rodríguez, H.; Campos, G.; Curiel, J.A.; Ascaso, C.; Carrascosa, A.V.; Prieto, A.; Rivas, B.D.L.; Muñoz, R.; De Felipe, F.L. Tannic Acid-Dependent Modulation of Selected Lactobacillus plantarum Traits Linked to Gastrointestinal Survival. PLoS ONE 2013, 8, e66473. [Google Scholar] [CrossRef] [Green Version]
- Reverón, I.; de las Rivas, B.; Muñoz, R.; López de Felipe, F. Genome-wide transcriptomic responses of a human isolate of Lactobacillus plantarum exposed to p-coumaric acid stress. Mol. Nutr. Food Res. 2012, 56, 1848–1859. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Goñi, I. Dietary Polyphenols and Human Gut Microbiota: A Review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Vaughan, E.E. Probiotic and Gut Lactobacilli and Bifidobacteria: Molecular Approaches to Study Diversity and Activity. Annu. Rev. Microbiol. 2009, 63, 269–290. [Google Scholar] [CrossRef]
- Esteban-Torres, M.; Reverón, I.; Plaza-Vinuesa, L.; Rivas, B.D.L.; Muñoz, R.; De Felipe, F.L. Transcriptional Reprogramming at Genome-Scale of Lactobacillus plantarum WCFS1 in Response to Olive Oil Challenge. Front. Microbiol. 2017, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Reverón, I.; Rivas, B.D.L.; Matesanz, R.; Muñoz, R.; De Felipe, F.L. Molecular adaptation of Lactobacillus plantarum WCFS1 to gallic acid revealed by genome-scale transcriptomic signature and physiological analysis. Microb. Cell Factories 2015, 14, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverón, I.; Jiménez, N.; Curiel, J.A.; Peñas, E.; De Felipe, F.L.; Rivas, B.D.L.; Muñoz, R. Differential Gene Expression by Lactobacillus plantarum WCFS1 in Response to Phenolic Compounds Reveals New Genes Involved in Tannin Degradation. Appl. Environ. Microbiol. 2017, 83, e03387-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverón, I.; Plaza-Vinuesa, L.; Franch, M.; Rivas, B.D.L.; Muñoz, R.; De Felipe, F.L. Transcriptome-Based Analysis in Lactobacillus plantarum WCFS1 Reveals New Insights into Resveratrol Effects at System Level. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Santamaría, L.; Reverón, I.; Plaza-Vinuesa, L.; Oliveros, J.C.; Rivas, B.D.L.; Muñoz, R.; De Felipe, F.L. Oleuropein Transcriptionally Primes Lactobacillus plantarum to Interact with Plant Hosts. Front. Microbiol. 2019, 10, 2177. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Crecchio, C.; De Virgilio, C.; De Angelis, M.; Gobbetti, M. Transcriptional reprogramming and phenotypic switching associated with the adaptation of Lactobacillus plantarum C2 to plant niches. Sci. Rep. 2016, 6, 27392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filannino, P.; De Angelis, M.; Di Cagno, R.; Gozzi, G.; Riciputi, Y.; Gobbetti, M. How Lactobacillus plantarum shapes its transcriptome in response to contrasting habitats. Environ. Microbiol. 2018, 20, 3700–3716. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Saulnier, D.M.A.; Molenaar, D.; De Vos, W.M.; Gibson, G.R.; Kolida, S. Identification of Prebiotic Fructooligosaccharide Metabolism in Lactobacillus plantarum WCFS1 through Microarrays. Appl. Environ. Microbiol. 2007, 73, 1753–1765. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Smyth, G.K.; Speed, T.; Speed, T.P. Normalization of cDNA microarray data. Methods 2003, 31, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; De Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, R.P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Boil. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [Green Version]
- Groot, M.N.N.; Klaassens, E.; De Vos, W.M.; Delcour, J.; Hols, P.; Kleerebezem, M. Genome-based in silico detection of putative manganese transport systems in Lactobacillus plantarum and their genetic analysis. Microbiology 2005, 151, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L.M.; Molenaar, D.; Wels, M.; Teusink, B.; Bron, P.A.; De Vos, W.M.; Smid, E.J. Thioredoxin reductase is a key factor in the oxidative stress response of Lactobacillus plantarum WCFS1. Microb. Cell Factories 2007, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A Universal Defense Against Antibiotics in Bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Tinajero-Trejo, M.; Jesse, H.E.; Poole, R.K. Gasotransmitters, poisons, and antimicrobials: It’s a gas, gas, gas! F1000Prime Rep. 2013, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Hassett, D.J.; Imlay, J.A. Bactericidal Antibiotics and Oxidative Stress: A Radical Proposal. ACS Chem. Boil. 2007, 2, 708–710. [Google Scholar] [CrossRef] [Green Version]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.-Y.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free. Radic. Boil. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Z.; Feng, Z.; Hao, J.; Shen, W.; Li, X.; Sun, L.; Sharman, E.; Wang, Y.; Wertz, K.; et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1089–1098. [Google Scholar] [CrossRef]
- Cho, K.H. The Structure and Function of the Gram-Positive Bacterial RNA Degradosome. Front. Microbiol. 2017, 8, 1866. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Malla, S.; Shin, B.; Li, J.M. Battle against RNA oxidation: Molecular mechanisms for reducing oxidized RNA to protect cells. Wiley Interdiscip. Rev. RNA 2013, 5, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Potrykus, K.; Cashel, M. (p)ppGpp: Still Magical? Annu. Rev. Microbiol. 2008, 62, 35–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Inouye, M. MazG, a Nucleoside Triphosphate Pyrophosphohydrolase, Interacts with Era, an Essential GTPase in Escherichia coli. J. Bacteriol. 2002, 184, 5323–5329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutte, C.C.; Crosson, S. Bacterial lifestyle shapes stringent response activation. Trends Microbiol. 2013, 21, 174–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.; Tenson, T.; Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Genet. 2015, 13, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Bouhss, A.; Crouvoisier, M.; Blanot, D.; Mengin-Lecreulx, D. Purification and Characterization of the Bacterial MraY Translocase Catalyzing the First Membrane Step of Peptidoglycan Biosynthesis. J. Boil. Chem. 2004, 279, 29974–29980. [Google Scholar] [CrossRef] [Green Version]
- Koskenniemi, K.; Laakso, K.; Koponen, J.; Kankainen, M.; Greco, D.; Auvinen, P.; Savijoki, K.; Nyman, T.A.; Surakka, A.; Salusjärvi, T.; et al. Proteomics and transcriptomics characterization of bile stress response in probiotic Lactobacillus rhamnosus GG. Mol. Cell. Proteom. 2010, 10. [Google Scholar] [CrossRef] [Green Version]
- Marsilio, V.; Campestre, C.; Lanza, B.; De Angelis, M. Sugar and polyol compositions of some European olive fruit varieties (Olea europaea L.) suitable for table olive purposes. Food Chem. 2001, 72, 485–490. [Google Scholar] [CrossRef]
- Poolman, B.; Molenaar, D.; Smid, E.J.; Ubbink, T.; Abee, T.; Renault, P.P.; Konings, W.N. Malolactic fermentation: Electrogenic malate uptake and malate/lactate antiport generate metabolic energy. J. Bacteriol. 1991, 173, 6030–6037. [Google Scholar] [CrossRef] [Green Version]
- Majchrzykiewicz, J.A.; Kuipers, O.P.; Bijlsma, J.J.E. Generic and Specific Adaptive Responses of Streptococcus pneumoniae to Challenge with Three Distinct Antimicrobial Peptides, Bacitracin, LL-37, and Nisin. Antimicrob. Agents Chemother. 2009, 54, 440–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rietkötter, E.; Hoyer, D.; Mascher, T. Bacitracin sensing in Bacillus subtilis. Mol. Microbiol. 2008, 68, 768–785. [Google Scholar] [CrossRef] [PubMed]
- Salzberg, L.I.; Luo, Y.; Hachmann, A.-B.; Mascher, T.; Helmann, J.D. The Bacillus subtilis GntR Family Repressor YtrA Responds to Cell Wall Antibiotics. J. Bacteriol. 2011, 193, 5793–5801. [Google Scholar] [CrossRef] [Green Version]
- Bolhuis, H.; van Veen, H.W.; Poolman, B.; Driessen, A.J.; Konings, W.N. Mechanisms of multidrug transporters. FEMS Microbiol. Rev. 1997, 21, 55–84. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reverón, I.; Plaza-Vinuesa, L.; Santamaría, L.; Oliveros, J.C.; de las Rivas, B.; Muñoz, R.; López de Felipe, F. Transcriptomic Evidence of Molecular Mechanisms Underlying the Response of Lactobacillus plantarum WCFS1 to Hydroxytyrosol. Antioxidants 2020, 9, 442. https://doi.org/10.3390/antiox9050442
Reverón I, Plaza-Vinuesa L, Santamaría L, Oliveros JC, de las Rivas B, Muñoz R, López de Felipe F. Transcriptomic Evidence of Molecular Mechanisms Underlying the Response of Lactobacillus plantarum WCFS1 to Hydroxytyrosol. Antioxidants. 2020; 9(5):442. https://doi.org/10.3390/antiox9050442
Chicago/Turabian StyleReverón, Inés, Laura Plaza-Vinuesa, Laura Santamaría, Juan Carlos Oliveros, Blanca de las Rivas, Rosario Muñoz, and Félix López de Felipe. 2020. "Transcriptomic Evidence of Molecular Mechanisms Underlying the Response of Lactobacillus plantarum WCFS1 to Hydroxytyrosol" Antioxidants 9, no. 5: 442. https://doi.org/10.3390/antiox9050442