Abstract
Background
Postural instability is a disease milestone signaling advanced disease.
Objectives
To estimate the onset of postural instability in monogenic parkinsonisms.
Methods
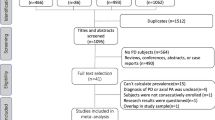
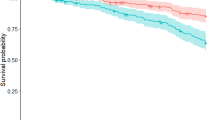
We systematically reviewed studies (PubMed 1996–2017) in SNCA, PRKN, PINK1, DJ-1, LRRK2, ATP13A2, FBXO7, VPS35, DNAJC6, or SYNJ1-related monogenic parkinsonisms, with documented postural instability. Genes with ≥ 15 patients were included in an individual-patient meta-analysis and compared with a retrospectively collected sporadic Parkinson’s disease cohort from our center. The primary outcome measure was the progression-free survival from postural instability using Kaplan–Meier survival curves. Cox proportional hazards analyses were summarized using hazards ratio (HR).
Results
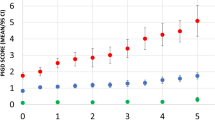
Of 2085 eligible studies, 124 met full criteria (636 patients) for the systematic review, whereas a total of 871 subjects (270 from sporadic cohort, 601 monogenic parkinsonisms) were included in the individual-patient meta-analysis. Postural instability was reported in 80% of DJ-1, 40% of PRKN, 39% of PINK1, 34% of ATP13A2, 31% of LRRK2, and 29% of SNCA patients. Progression-free survival from postural instability at 10 years after disease onset was longest in ATP13A2 (97%) and shortest in SNCA (50%). Halfway between these two extremes were PRKN (88%), PINK1 (87%), and LRRK2 (81%), similar to sporadic Parkinson’s disease (72%). Higher risk of postural instability was observed in SNCA (HR = 3.2, p = 0.007) and DJ-1 (HR = 3.96, p = 0.001) compared to sporadic Parkinson’s disease. Young age at onset in PINK1 and female sex in LRRK2 were associated with a decreased risk of postural instability.
Conclusions
Monogenic parkinsonisms exhibit differential timelines to postural instability, informing prognostic counseling and interpretation of future genotype-specific treatment trials.


Similar content being viewed by others
References
Hernandez DG, Reed X, Singleton AB (2016) Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J Neurochem 139:59–74. https://doi.org/10.1111/jnc.13593
Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D et al (2017) Past, present, and future of Parkinson's disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord 32:1264–1310. https://doi.org/10.1002/mds.27115
Klein C, Westenberger A (2012) Genetics of Parkinson's disease. Cold Spring Harb Perspect Med 2:a008888. https://doi.org/10.1101/cshperspect.a008888
Behrens MI, Brüggemann N, Chana P, Venegas P, Kägi M, Parrao T et al (2010) Clinical spectrum of Kufor-Rakeb syndrome in the Chilean kindred with ATP13A2 mutations. Mov Disord 25:1929–1937. https://doi.org/10.1002/mds.22996
Schneider SA, Paisan-Ruiz C, Quinn NP, Lees AJ, Houlden H, Hardy J (2010) ATP13A2 mutations (PARK9) cause neurodegeneration with brain iron accumulation. Mov Disord 25:979–984. https://doi.org/10.1002/mds.22947
Eiberg H, Hansen L, Korbo L, Nielsen IM, Svenstrup K, Bech S (2012) Novel mutation in ATP13A2 widens the spectrum of Kufor-Rakeb syndrome (PARK9). Clin Genet 82:256–263. https://doi.org/10.1111/j.1399-0004.2011.01745.x
Puschmann A (2013) Monogenic Parkinson's disease and parkinsonism: clinical phenotypes and frequencies of known mutations. Parkinsonism Relat Disord 19:407–415
Lesage S, Brice A (2009) Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet 18:R48–59. https://doi.org/10.1093/hmg/ddp012
Samaranch L, Lorenzo-Betancor O, Arbelo JM, Ferrer I, Lorenzo E, Irigoyen J et al (1128e) PINK1-linked parkinsonism is associated with Lewy body pathology. Brain 133:1128e42. https://doi.org/10.1371/journal.pone.0036199
Kempster PA, Williams DR, Selikhova M, Holton J, Revesz T, Lees AJ (2007) Patterns of levodopa response in Parkinson's disease: a clinico-pathological study. Brain 130:2123–2128
Kempster PA, O'Sullivan SS, Holton JL, Revesz T, Lees AJ (2010) Relationships between age and late progression of Parkinson's disease: a clinico-pathological study. Brain 133:1755–1762. https://doi.org/10.1093/brain/awq059
O'Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL et al (2008) Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 131:1362–1372. https://doi.org/10.1093/brain/awn065
Evans JR, Mason SL, Williams-Gray CH, Foltynie T, Brayne C, Robbins TW et al (2011) The natural history of treated Parkinson's disease in an incident, community based cohort. J Neurol Neurosurg Psychiatry 82:1112–1118. https://doi.org/10.1136/jnnp.2011.240366
Halliday G, Lees AJ, Stern M (2011) Milestones in Parkinson's disease–clinical and pathologic features. Mov Disord 26:1015–1021. https://doi.org/10.1002/mds.23669
De Pablo-Fernandez E, Tur C, Revesz T, Lees AJ, Holton JL, Warner TT (2017) Association of Autonomic Dysfunction With Disease Progression and Survival in Parkinson Disease. JAMA Neurol 74:970–976. https://doi.org/10.1001/jamaneurol.2017.1125
Wielinski CL, Erickson-Davis C, Wichmann R, Walde-Douglas M, Parashos SA (2005) Falls and injuries resulting from falls among patients with Parkinson's disease and other parkinsonian syndromes. Mov Disord 20:410–415
Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N (2005) Falls in outpatients with Parkinson's disease: frequency, impact and identifying factors. J Neurol 252:1310–1315
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) PRISMA Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al (2000) Thacker, Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
HoehnYahr MM (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
De Pablo-Fernández E, Lees AJ, Holton JL, Warner TT (2019) Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol 76:470–479. https://doi.org/10.1001/jamaneurol.2018.4377
Bjornestad A, Pedersen KF, Tysnes OB, Alves G (2017) Clinical milestones in Parkinson's disease: a 7-year population-based incident cohort study. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2017.05.025
Skorvanek M, Martinez-Martin P, Kovacs N, Rodriguez-Violante M, Corvol JC, Taba P et al (2017) Differences in MDS-UPDRS scores based on Hoehn and Yahr stage and disease duration. Mov Disord Clin Pract 4:536–544. https://doi.org/10.1002/mdc3.12476
Folstein MF, Folstein SE, McHugh PR (1975) "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA et al (2007) Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord 22:2314–2324
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y et al (2007) Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 22:1689–1707
Williams DR, Lees AJ (2005) Visual hallucinations in the diagnosis of idiopathic Parkinson's disease: a retrospective autopsy study. Lancet Neurol 4:605–610
Wang G, Huang Y, Chen W, Chen S, Wang Y, Xiao Q et al (2016) Variants in the SNCA gene associate with motor progression while variants in the MAPT gene associate with the severity of Parkinson's Disease. Parkinsonism Relat Disord 24:89e84. https://doi.org/10.1016/j.parkreldis.2015.12.018
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
DerSimonian R, Laird N (2015) Meta-analysis in clinical trials revisited. Contemp Clin Trials 45:139–145
Shulman LM, Gruber-Baldini AL, Anderson KE, Vaughan CG, Reich SG, Fishman PS et al (2008) The evolution of disability in Parkinson disease. Mov Disord 23:790–796
Wang G, Huang Y, Chen W, Chen S, Wang Y, Xiao Q et al (2016) Variants in the SNCA gene associate with motor progression while variants in the MAPT gene associate with the severity of Parkinson's disease. Parkinsonism Relat Disord 24:89–94
Poulopoulos M, Levy OA, Alcalay RN (2012) The neuropathology of genetic Parkinson’s disease. Mov Disord 27:831–842. https://doi.org/10.1002/mds.24962
Ahlskog JE (2009) Parkin and PINK1 parkinsonism may represent nigral mitochondrial cytopathies distinct from Lewy body Parkinson’s disease. Parkinonism Relat Disord 15:721–727. https://doi.org/10.1016/j.parkreldis.2009.09.010
Wider C, Dickson DW, Wszolek ZK (2010) Leucine-rich repeat kinase 2 gene- associated disease: redefining genotype-phenotype correlation. Neurodegener Dis 7:175–179. https://doi.org/10.1159/000289232
Park JS, Blair NF, Sue CM (2015) The role of ATP13A2 in Parkinson's disease: clinical phenotypes and molecular mechanisms. Mov Disord 30:770–779
Ahrweiller K, Houvenaghel JF, Riou A, Drapier S, Sauleau P, Haegelen C et al (2019) Postural instability and gait disorders after subthalamic nucleus deep brain stimulation in Parkinson’s disease: a PET study. J Neurol 266:2764–2771. https://doi.org/10.1007/s00415-019-09482-y
Galna B, Lord S, Burn DJ, Rochester L (2015) Progression of gait dysfunction in incident Parkinson's disease: Impact of medication and phenotype. Mov Disord 30:359–367
Riboldi GM, Di Fonzo AB (2019) GBA, Gaucher disease, and Parkinson's disease: from genetic to clinic to new therapeutic approaches. Cells. https://doi.org/10.3390/cells8040364
Acknowledgements
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
(1) Research project: A. conception, B. organization, C. execution; (2) statistical analysis: A. design, B. execution, C. review and critique; (3) manuscript: A. writing of the first draft, B. review and critique. LM, JAV, AS: 1A, 1B, 1C, 2A, 2B, 3A. AD: 2A, 2B, 2C. EGK, DP, MM, AF: 1C, 3B. AM, AF, IFM, MAK: 3B. AJE: 1A, 1B, 1C, 3B. All the co-authors listed above gave their final approval for this manuscript version.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Marsili reports no disclosure. Dr. Vizcarra reports no disclosure. Dr. Sturchio reports no disclosure. Dr. Dwivedi reports no disclosure. Ms. Keeling reports no disclosure. Dr. Patel reports no disclosure. Dr. Mishra reports no disclosure. Dr. Farooqi reports no disclosure. Dr. Merola is supported by NIH (KL2 TR001426) and has received speaker honoraria from CSL Behring, and Cynapsus Therapeutics. He has received grant support from Lundbeck. Dr. Fasano has received grant support from the University of Toronto, the McLaughlin Centre and the Michael J. Fox Foundation; he received speaking honoraria from UCB pharma, Medtronic, Boston Scientific, Abbvie, Novartis, Chiesi pharmaceutical, Ipsen, TEVA, the American Academy of Neurology, and the Movement Disorders Society; he receives publishing royalties from Springer; he is in an advisory board for Abbvie and Ipsen and provided consultancies for Sunovion, UCB pharma, Medtronic, Boston Scientific, and Abbvie. Dr. Kauffman is an employee of the CONICET. He has received grant support from Ministry of Science and Technology of Argentina and Ministry of Health of Buenos Aires. Dr. Mata receives funding support from the Department of Veterans Affairs, National Institute of Health, Parkinson’s Foundation and American Parkinson Disease Association. Dr. Espay has received grant support from the NIH and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, Neuroderm, Neurocrine, Amneal, Adamas, Acadia, Acorda, InTrance, Sunovion, Lundbeck, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from USWorldMeds, Acadia, and Sunovion.
Ethical approval
The study has been approved by the local ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marsili, L., Vizcarra, J.A., Sturchio, A. et al. When does postural instability appear in monogenic parkinsonisms? An individual-patient meta-analysis. J Neurol 268, 3203–3211 (2021). https://doi.org/10.1007/s00415-020-09892-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09892-3