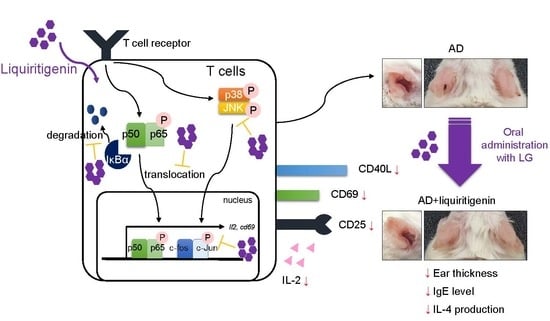
Oral Administration of Liquiritigenin Confers Protection from Atopic Dermatitis through the Inhibition of T Cell Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Mice
2.3. Plant Materials
2.4. Extraction and Isolation
2.5. Condition of High-Performance Liquid Chromatography (HPLC) Analysis
2.6. Reagent and Antibodies
2.7. MTT Assay
2.8. T Cell Stimulation
2.9. ELISA
2.10. Flow Cytometry
2.11. Western Blot Analysis
2.12. NFκB Translocation Analysis by Western Blot
2.13. AD induction in Mice Ear
2.14. Histological Analysis
2.15. Isolation of CD4+ T Cells
2.16. Statistics
3. Results
3.1. Liquiritigenin Does not Induce Cellular Apoptosis
3.2. Treatment of Jurkat T Cells with Liquiritigenin Blocks T Cell Activation
3.3. Liquiritigenin Reduces the Expression of Surface Molecules on Activated T Cells
3.4. Liquiritigenin Inhibits p65 Translocation and MAPK Signaling Pathways in Activated T Cells
3.5. Oral Administration of Liquiritigenin Mitigates Atopic Dermatitis in Mice
3.6. Oral Administration of Liquiritigenin on AD Mice Systemically Decreases the Expression of Effector Cytokines from Effector T Cells
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Li, R.W.; David Lin, G.; Myers, S.P.; Leach, D.N. Anti-inflammatory activity of Chinese medicinal vine plants. J. Ethnopharmacol. 2003, 85, 61–67. [Google Scholar] [CrossRef]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; de la Cruz, M.; Monteiro, M.C.; Melguizo, Á.; et al. Anti-fungal and anti-bacterial activities of ethanol extracts of selected traditional Chinese medicinal herbs. Asian Pac. J. Trop. Med. 2013, 6, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-Y.; Wang, D.-M.; Loo, T.Y.; Cheng, Y.; Chen, L.-L.; Shen, J.-G.; Yang, D.-P.; Chow, L.W.-C.; Guan, X.-Y.; Chen, J.-P. Spatholobus suberectus inhibits cancer cell growth by inducing apoptosis and arresting cell cycle at G2/M checkpoint. J. Ethnopharmacol. 2011, 133, 751–758. [Google Scholar] [CrossRef]
- Chen, H.L.; Yang, J.; Fu, Y.F.; Meng, X.N.; Zhao, W.D.; Hu, T.J. Effect of total flavonoids of Spatholobus suberectus Dunn on PCV2 induced oxidative stress in RAW264.7 cells. BMC Complement. Altern. Med. 2017, 17, 244. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.Y.; Ha, J.Y.; Kim, K.M.; Jung, Y.S.; Jung, J.C.; Oh, S. Anti-inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules 2015, 20, 13041–13054. [Google Scholar] [CrossRef]
- Kong, L.D.; Zhang, Y.; Pan, X.; Tan, R.X.; Cheng, C.H.K. Inhibition of xanthine oxidase by liquiritigenin and isoliquiritigenin isolated from Sinofranchetia chinensis. Cell. Mol. Life Sci. 2000, 57, 500–505. [Google Scholar] [CrossRef]
- Khamsan, S.; Liawruangrath, S.; Teerawutkulrag, A.; Pyne, S.G.; Garson, M.J.; Liawruangrath, B. The isolation of bioactive flavonoids from Jacaranda obtusifolia H. B. K. ssp. rhombifolia (G. F. W. Meijer) Gentry. Acta Pharm. 2012, 62, 181–190. [Google Scholar]
- Jahromi, M.A.F.; Ray, A.B.; Chansouria, J.P.N. Antihyperlipidemic effect of flavonoids from pterocarpus marsupium. J. Nat. Prod. 1993, 56, 989–994. [Google Scholar] [CrossRef]
- Xie, X.W. Liquiritigenin attenuates cardiac injury induced by high fructose-feeding through fibrosis and inflammation suppression. Biomed. Pharmacother. 2017, 86, 694–704. [Google Scholar] [CrossRef]
- Meng, F.C.; Lin, J.K. Liquiritigenin inhibits colorectal cancer proliferation, invasion, and epithelial-to-mesenchymal transition by decreasing expression of runt-related transcription factor 2. Oncol. Res. 2019, 27, 139–146. [Google Scholar] [CrossRef]
- Shi, C.; Wu, H.; Xu, K.; Cai, T.; Qin, K.; Wu, L.; Cai, B. Liquiritigenin-loaded submicron emulsion protects against doxorubicin-induced cardiotoxicity via antioxidant, anti-inflammatory, and anti-apoptotic activity. Int. J. Nanomed. 2020, 15, 1101–1115. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Zhang, C.; Zhang, J.; Kai, G.; Lu, B.; Huang, Z.; Ji, L. The involvement of DAMPs-mediated inflammation in cyclophosphamide-induced liver injury and the protection of liquiritigenin and liquiritin. Eur. J. Pharmacol. 2019, 856, 172421. [Google Scholar] [CrossRef]
- Tu, C.; Ma, Y.; Song, M.; Yan, J.; Xiao, Y.; Wu, H. Liquiritigenin inhibits IL-1β-induced inflammation and cartilage matrix degradation in rat chondrocytes. Eur. J. Pharmacol. 2019, 858, 172445. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Yu, H.; Ma, F.; Wu, J.; Zhang, X. Liquiritigenin Protects Rats from Carbon Tetrachloride Induced Hepatic Injury through PGC-1α Pathway. Evid. Based Complement. Altern. Med. 2015, 2015, 649568. [Google Scholar] [CrossRef]
- Purtic, B.; Pitcher, L.A.; Van Oers, N.S.C.; Wülfing, C. T cell receptor (TCR) clustering in the immunological synapse integrates TCR and costimulatory signaling in selected T cells. Proc. Natl. Acad. Sci. USA 2005, 102, 2904–2909. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, S.F.; Ramsdell, F.; Alderson, M.R. The activation antigen CD69. Stem Cells 1994, 12, 456–465. [Google Scholar] [CrossRef]
- Crawley, J.B.; Rawlinson, L.; Lali, F.V.; Page, T.H.; Saklatvala, J.; Foxwell, B.M.J. T Cell Proliferation in Response to Interleukins 2 and 7 Requires p38MAP Kinase Activation. J. Biol. Chem. 1997, 272, 15023–15027. [Google Scholar] [CrossRef] [Green Version]
- Sojka, D.K.; Bruniquel, D.; Schwartz, R.H.; Singh, N.J. IL-2 Secretion by CD4+ T Cells In Vivo Is Rapid, Transient, and Influenced by TCR-Specific Competition. J. Immunol. 2004, 172, 6136–6143. [Google Scholar] [CrossRef] [Green Version]
- Bajnok, A.; Ivanova, M.; Rigó, J.; Toldi, G. The Distribution of Activation Markers and Selectins on Peripheral T Lymphocytes in Preeclampsia. Mediat. Inflamm. 2017, 2017, 8045161. [Google Scholar] [CrossRef]
- Grewal, I.S.; Flavell, R.A. The Role of CD40 Ligand in Costimulation and T-Cell Activation. Immunol. Rev. 1996, 153, 85–106. [Google Scholar] [CrossRef]
- Abernethy, N.J.; Hagan, C.; Tan, P.L.J.; Watson, J.D. Dysregulated expression of CD69 and IL-2 receptor α and β chains on CD8+ T lymphocytes in flaky skin mice. Immunol. Cell Biol. 2000, 78, 596–602. [Google Scholar] [CrossRef]
- Grammatikos, A.P. The genetic and environmental basis of atopic diseases. Ann. Med. 2008, 40, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.M.; Nomura, I.; Hamid, Q.A.; Leung, D.Y.M.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis Find the latest version: Science in medicine New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Bieber, T. Atopic dermatitis. Lancet 2003, 361, 151–160. [Google Scholar] [CrossRef]
- Akdis, M.; Trautmann, A.; Blaser, K.; Akdis, C.A. T cells and effector mechanisms in the pathogenesis of atopic dermatitis. Curr. Allergy Asthma Rep. 2002, 2, 1–3. [Google Scholar] [CrossRef]
- Auriemma, M.; Vianale, G.; Amerio, P.; Reale, M. Cytokines and T cells in atopic dermatitis. Eur. Cytokine Netw. 2013, 24, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.N.; Qu, X.B.; Guan, S.H.; Xu, P.P.; Shi, Y.Y.; Guo, D.A. Chemical constituents of Spatholobus suberectus. Chin. J. Nat. Med. 2012, 10, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Choi, E.-J.; Choi, H.; Lee, K.-S.; Kim, H.-R.; Na, B.-R.; Kwon, M.-S.; Jeong, G.-S.; Choi, H.G.; Choi, E.Y.; et al. Oral Administration of 4-Hydroxy-3-Methoxycinnamaldehyde Attenuates Atopic Dermatitis by Inhibiting T Cell and Keratinocyte Activation. PLoS ONE 2015, 10, e0144521. [Google Scholar] [CrossRef]
- Mohapatra, D.K.; Das, P.P.; Sai Reddy, D.; Yadav, J.S. First total syntheses and absolute configuration of rugulactone and 6(R)-(4′-oxopent-2′-enyl)-5,6-dihydro-2H-pyran-2-one. Tetrahedron Lett. 2009, 50, 5941–5944. [Google Scholar] [CrossRef]
- Mohapatra, D.K.; Reddy, D.S.; Janaki Ramaiah, M.; Ghosh, S.; Pothula, V.; Lunavath, S.; Thomas, S.; Pushpa Valli, S.N.C.V.L.; Bhadra, M.P.; Yadav, J.S. Rugulactone derivatives act as inhibitors of NF-κB activation and modulates the transcription of NF-κB dependent genes in MDA-MB-231cells. Bioorg. Med. Chem. Lett. 2014, 24, 1389–1396. [Google Scholar] [CrossRef]
- Schmitz, M.L.; Krappmann, D. Controlling NF-κB activation in T cells by costimulatory receptors. Cell Death Differ. 2006, 13, 834–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-S.; Choi, E.-J.; Lee, K.-S.; Kim, H.-R.; Na, B.-R.; Kwon, M.-S.; Jeong, G.-S.; Choi, H.G.; Choi, E.Y.; Jun, C.-D. Oral Administration of p-Hydroxycinnamic Acid Attenuates Atopic Dermatitis by Downregulating Th1 and Th2 Cytokine Production and Keratinocyte Activation. PLoS ONE 2016, 11, e0150952. [Google Scholar] [CrossRef] [Green Version]
- Masopust, D.; Schenkel, J.M. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 2013, 13, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Dimeloe, S.; Burgener, A.V.; Grählert, J.; Hess, C. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology 2017, 150, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xie, S.; Liu, C.; Wu, Y.; Liu, Y.; Cai, Y. Inhibitory effect of liquiritigenin on migration via downregulation ProMMP-2 and PI3K/Akt signaling pathway in human lung adenocarcinoma A549 cells. Nutr. Cancer 2012, 64, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L. T-cell activation through immunological synapses and kinapses. Immunol. Rev. 2008, 221, 77–89. [Google Scholar] [CrossRef]
- Kawabe, T.; Matsushima, M.; Hashimoto, N.; Imaizumi, K.; Hasegawa, Y. CD40/CD40 ligand interactions in immune responses and pulmonary immunity. Nagoya J. Med. Sci. 2011, 73, 69–78. [Google Scholar]
- Hernandez, M.G.H.; Shen, L.; Rock, K.L. CD40-CD40 Ligand Interaction between Dendritic Cells and CD8+ T Cells Is Needed to Stimulate Maximal T Cell Responses in the Absence of CD4+ T Cell Help. J. Immunol. 2007, 178, 2844–2852. [Google Scholar] [CrossRef] [Green Version]
- Boyman, O.; Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012, 12, 180–190. [Google Scholar] [CrossRef]
- Shatrova, A.N.; Mityushova, E.V.; Vassilieva, I.O.; Aksenov, N.D.; Zenin, V.V.; Nikolsky, N.N.; Marakhova, I.I. Time-dependent regulation of IL-2R α-chain (CD25) expression by TCR signal strength and IL-2-induced STAT5 signaling in activated human blood T lymphocytes. PLoS ONE 2016, 11, e0167215. [Google Scholar] [CrossRef] [Green Version]
- Bax, H.J.; Keeble, A.H.; Gould, H.J. Cytokinergic IgE action in mast cell activation. Front. Immunol. 2012, 3, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buddenkotte, J.; Maurer, M.; Steinhoff, M. Histamine and Antihistamines in Atopic Dermatitis. In Histamine in Inflammation; Springer: Boston, MA, USA, 2010; pp. 73–80. [Google Scholar]
- Hellman, L.T.; Akula, S.; Thorpe, M.; Fu, Z. Tracing the origins of IgE, mast cells, and allergies by studies of wild animals. Front. Immunol. 2017, 8, 1749. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-S.; Kim, E.-N.; Jeong, G.-S. Oral Administration of Liquiritigenin Confers Protection from Atopic Dermatitis through the Inhibition of T Cell Activation. Biomolecules 2020, 10, 786. https://doi.org/10.3390/biom10050786
Lee H-S, Kim E-N, Jeong G-S. Oral Administration of Liquiritigenin Confers Protection from Atopic Dermatitis through the Inhibition of T Cell Activation. Biomolecules. 2020; 10(5):786. https://doi.org/10.3390/biom10050786
Chicago/Turabian StyleLee, Hyun-Su, Eun-Nam Kim, and Gil-Saeng Jeong. 2020. "Oral Administration of Liquiritigenin Confers Protection from Atopic Dermatitis through the Inhibition of T Cell Activation" Biomolecules 10, no. 5: 786. https://doi.org/10.3390/biom10050786