Abstract
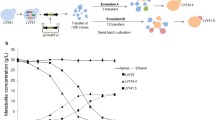
As the effects of climate change become apparent, metabolic engineers and synthetic biologists are exploring sustainable sources for transportation fuels. The design and engineering of microorganisms to produce gasoline, diesel, and jet fuel compounds from renewable feedstocks can significantly reduce our dependence on fossil fuels as well as lower the emissions of greenhouse gases. Over the past 2 decades, a considerable amount of work has led to the development of microbial strains for the production of advanced fuel compounds from both C5 and C6 sugars. In this work, we combined two strategies—adaptive laboratory evolution and rational metabolic engineering—to improve the yeast Saccharomyces cerevisiae’s ability to utilize d-xylose, a major C5 sugar in biomass, and produce the advanced biofuel isobutanol. Whole genome resequencing of several evolved strains followed by reverse engineering identified two single nucleotide mutations, one in CCR4 and another in TIF1, that improved the yeast’s specific growth rate by 23% and 14%, respectively. Neither one of these genes has previously been implicated to play a role in utilization of d-xylose. Fine-tuning the expression levels of the bottleneck enzymes in the isobutanol pathway further improved the evolved strain’s isobutanol titer to 92.9 ± 4.4 mg/L (specific isobutanol production of 50.2 ± 2.6 mg/g DCW), a 90% improvement in titer and a 110% improvement in specific production over the non-evolved strain. We hope that our work will set the stage for an economic route to the advanced biofuel isobutanol and enable efficient utilization of xylose-containing biomass.






Similar content being viewed by others
References
Liao JC, Mi L, Pontrelli S, Luo S (2016) Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat Rev Microbiol 14:288–304
Keasling JD (2010) Manufacturing molecules through metabolic engineering. Science 330:1355–1358
Peralta-Yahya PP, Zhang F, del Cardayre SB, Keasling JD (2012) Microbial engineering for the production of advanced biofuels. Nature 488:320–328. https://doi.org/10.1038/nature11478
Kung Y, Runguphan W, Keasling JD (2012) From fields to fuels: recent advances in the microbial production of biofuels. ACS Synth Biol 1:498–513. https://doi.org/10.1021/sb300074k
Lee JW, Na D, Park JM et al (2012) Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat Chem Biol 8:536–546
Peralta-Yahya PP, Ouellet M, Chan R et al (2011) Identification and microbial production of a terpene-based advanced biofuel. Nat Commun 2:483. https://doi.org/10.1038/ncomms1494
Atsumi S, Hanai T, Liao JC (2008) Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89. https://doi.org/10.1038/nature06450
Choi YJ, Lee SY (2013) Microbial production of short-chain alkanes. Nature 502:571–574. https://doi.org/10.1038/nature12536
Buijs NA, Zhou YJ, Siewers V, Nielsen J (2015) Long-chain alkane production by the yeast Saccharomyces cerevisiae. Biotechnol Bioeng 112:1275–1279. https://doi.org/10.1002/bit.25522
Steen EJ, Kang Y, Bokinsky G et al (2010) Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463:559–562. https://doi.org/10.1038/nature08721
Runguphan W, Keasling JD (2013) Metabolic engineering of Saccharomyces cerevisiae for production of fatty acid-derived biofuels and chemicals. Metab Eng. https://doi.org/10.1016/j.ymben.2013.07.003
Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R (2011) Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. Nature 476:355–359. https://doi.org/10.1038/nature10333
Kwak S, Jin YS (2017) Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: a review and perspective. Microb Cell Fact 16:82
Brat D, Boles E (2013) Isobutanol production from d-xylose by recombinant Saccharomyces cerevisiae. FEMS Yeast Res 13:241–244. https://doi.org/10.1111/1567-1364.12028
Promdonkoy P, Siripong W, Downes JJ et al (2019) Systematic improvement of isobutanol production from d-xylose in engineered Saccharomyces cerevisiae. AMB Express 9:160. https://doi.org/10.1186/s13568-019-0885-3
Lane S, Zhang Y, Yun EJ et al (2020) Xylose assimilation enhances the production of isobutanol in engineered Saccharomyces cerevisiae. Biotechnol Bioeng 117:372–381. https://doi.org/10.1002/bit.27202
Zhang Y, Lane S, Chen J-M et al (2019) Xylose utilization stimulates mitochondrial production of isobutanol and 2-methyl-1-butanol in Saccharomyces cerevisiae. Biotechnol Biofuels 12:223. https://doi.org/10.1186/s13068-019-1560-2
Dragosits M, Mattanovich D (2013) Adaptive laboratory evolution—principles and applications for biotechnology. Microb Cell Fact 12:64. https://doi.org/10.1186/1475-2859-12-64
Caspeta L, Chen Y, Ghiaci P et al (2014) Altered sterol composition renders yeast thermotolerant. Science 346:75–78. https://doi.org/10.1126/science.1258137
van Maris AJA, Geertman J-MA, Vermeulen A et al (2004) Directed evolution of pyruvate decarboxylase-negative Saccharomyces cerevisiae, yielding a C2-independent, glucose-tolerant, and pyruvate-hyperproducing yeast. Appl Environ Microbiol 70:159–166. https://doi.org/10.1128/aem.70.1.159-166.2004
Davis López SA, Griffith DA, Choi B et al (2018) Evolutionary engineering improves tolerance for medium-chain alcohols in Saccharomyces cerevisiae. Biotechnol Biofuels 11:90. https://doi.org/10.1186/s13068-018-1089-9
Farzadfard F, Perli SD, Lu TK (2013) Tunable and multifunctional eukaryotic transcription factors based on CRISPR/Cas. ACS Synth Biol 2:604–613. https://doi.org/10.1021/sb400081r
DiCarlo JE, Norville JE, Mali P et al (2013) Genome engineering in Saccharomyces cerevisiae using CRISPR–Cas systems. Nucleic Acids Res 41:4336–4343. https://doi.org/10.1093/nar/gkt135
Gietz RD, Schiestl RH (2007) Frozen competent yeast cells that can be transformed with high efficiency using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2:1–4. https://doi.org/10.1038/nprot.2007.17
Gietz RD, Schiestl RH (2007) High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2:31–34. https://doi.org/10.1038/nprot.2007.13
Chen S, Zhou Y, Chen Y, Gu J (2018) Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25:1754–1760. https://doi.org/10.1093/bioinformatics/btp324
McKenna A, Hanna M, Banks E et al (2010) The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. https://doi.org/10.1101/gr.107524.110
Reider Apel A, d’Espaux L, Wehrs M et al (2016) A Cas9-based toolkit to program gene expression in Saccharomyces cerevisiae. Nucleic Acids Res 45:496–508. https://doi.org/10.1093/nar/gkw1023
Hegemann JH, Heick SB (2011) Delete and repeat: a comprehensive toolkit for sequential gene knockout in the budding yeast Saccharomyces cerevisiae. Methods Mol Biol 765:189–206. https://doi.org/10.1007/978-1-61779-197-0_12
Boeke JD, La Croute F, Fink GR (1984) A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. MGG Mol Gen Genet 197:345–346. https://doi.org/10.1007/BF00330984
Collart MA, Panasenko OO (2012) The Ccr4-Not complex. Gene 492:42–53. https://doi.org/10.1016/j.gene.2011.09.033
Miller JE, Reese JC (2012) Ccr4-Not complex: the control freak of eukaryotic cells. Crit Rev Biochem Mol Biol 47:315–333. https://doi.org/10.3109/10409238.2012.667214
Traven A, Hammet A, Tenis N et al (2005) Ccr4-not complex mRNA deadenylase activity contributes to DNA damage responses in Saccharomyces cerevisiae. Genetics 169:65–75. https://doi.org/10.1534/genetics.104.030940
Lenssen E, James N, Pedruzzi I et al (2005) The Ccr4-not complex independently controls both Msn2-dependent transcriptional activation—via a newly identified Glc7/Bud14 type I protein phosphatase module—and TFIID promoter distribution. Mol Cell Biol 25:488–498. https://doi.org/10.1128/MCB.25.1.488-498.2005
Azzouz N, Panasenko OO, Deluen C et al (2009) Specific roles for the Ccr4-not complex subunits in expression of the genome. RNA 15:377–383. https://doi.org/10.1261/rna.1348209
Cui Y, Ramnarain DB, Chiang Y-C et al (2008) Genome wide expression analysis of the CCR4-NOT complex indicates that it consists of three modules with the NOT module controlling SAGA-responsive genes. Mol Genet Genom 279:323–337. https://doi.org/10.1007/s00438-007-0314-1
Li Q, Imataka H, Morino S et al (1999) Eukaryotic translation initiation factor 4AIII (eIF4AIII) is functionally distinct from eIF4AI and eIF4AII. Mol Cell Biol 19:7336–7346. https://doi.org/10.1128/mcb.19.11.7336
Gupta N, Lorsch JR, Hinnebusch AG (2018) Yeast Ded1 promotes 48S translation preinitiation complex assembly in an mRNA-specific and eIF4F-dependent manner. Elife. https://doi.org/10.7554/eLife.38892
Schmid SR, Linder P (1991) Translation initiation factor 4A from Saccharomyces cerevisiae: analysis of residues conserved in the D-E-A-D family of RNA helicases. Mol Cell Biol 11:3463–3471. https://doi.org/10.1128/mcb.11.7.3463
Basquin J, Roudko VV, Rode M et al (2012) Architecture of the nuclease module of the yeast Ccr4-not complex: the Not1-Caf1-Ccr4 interaction. Mol Cell 48:207–218. https://doi.org/10.1016/j.molcel.2012.08.014
Obenauer JC, Cantley LC, Yaffe MB (2003) Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31:3635–3641. https://doi.org/10.1093/nar/gkg584
Blom N, Gammeltoft S, Brunak S (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294:1351–1362. https://doi.org/10.1006/jmbi.1999.3310
Ryan MD, Drew J (1994) Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J 13:928–933. https://doi.org/10.1002/j.1460-2075.1994.tb06337.x
Geier M, Fauland P, Vogl T, Glieder A (2015) Compact multi-enzyme pathways in P. pastoris. Chem Commun 51:1643–1646. https://doi.org/10.1039/C4CC08502G
Beekwilder J, van Rossum HM, Koopman F et al (2014) Polycistronic expression of a β-carotene biosynthetic pathway in Saccharomyces cerevisiae coupled to β-ionone production. J Biotechnol 192:383–392. https://doi.org/10.1016/j.jbiotec.2013.12.016
Liu Z, Chen O, Wall JBJ et al (2017) Systematic comparison of 2A peptides for cloning multi-genes in a polycistronic vector. Sci Rep 7:2193. https://doi.org/10.1038/s41598-017-02460-2
Acknowledgements
We thank Professor George Church (Harvard University) and Professor Timothy K. Lu (Massachusetts Institute of Technology) for the CRISPR/Cas9 plasmids. We thank Justin W. Henceroth for his critical reading of this manuscript.
Funding
This work conducted by the Thailand National Center for Genetic Engineering and Biotechnology (BIOTEC) was supported by the Thailand Research Fund (TRF) under Contract No. TRG6180006.
Author information
Authors and Affiliations
Contributions
PP carried out most of the plasmid construction, ALE experiment, strain construction and characterization. WM and VC contributed to the genome resequencing of the evolved strains. ST contributed to the experimental design and drafted the manuscript. WR conceived and designed the study, and drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Promdonkoy, P., Mhuantong, W., Champreda, V. et al. Improvement in d-xylose utilization and isobutanol production in S. cerevisiae by adaptive laboratory evolution and rational engineering. J Ind Microbiol Biotechnol 47, 497–510 (2020). https://doi.org/10.1007/s10295-020-02281-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-020-02281-9