Abstract
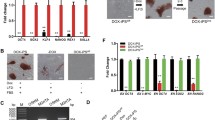
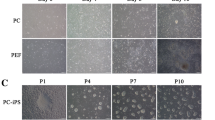
Researchers currently lack standardized porcine-specific markers that would aid in distinguishing the naïve and primed states of porcine embryonic stem cells (ESCs). Here, we converted naïve-like porcine ESCs (nESCs, established in our lab) into primed-state cells, and we proposed a set of molecular criteria for evaluating the naïve porcine ESCs by comparing the two cell states. The reverse-primed porcine ESCs (rpESCs) are phenotypically stable and karyotypically intact. Alkaline phosphatase positivity and the ability to form embryonic bodies suggest that rpESCs still retain the capacity for self-renewal. Lineage-associated genes, such as Cdx2, Sox17, Eomes, Foxa, Fgf5, and Pitx2, exhibited significant expression in rpESCs. Nonetheless, LIF/3i-grown porcine ESCs treated with the small molecular weight inhibitors CHIR99021, PD0325901, and SB431542 expressed the greatest number of pluripotency marker genes, including Oct4, Sox2, Nog, Dppa5, Nr0b1, and Klf4, and at higher levels than were observed in rpESCs. Despite their general trend toward higher expression of critical pluripotency factors, the nESCs showed downregulation of Tbx3, Nanog, and c-Myc, which are considered typical naïve factors in other species. Entry of the nESCs into the developmentally primed state was also associated with a marked reduction in Lin28 expression. These findings extend the knowledge of porcine pluripotency markers and provide a backdrop for future analysis of naïve porcine pluripotency.






Similar content being viewed by others
References
Boroviak T, Loos R, Lombard P, Okahara J, Behr R, Sasaki E, Nichols J, Smith A, Bertone P (2015) Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Dev Cell 35:366–382
Brevini TA, Pennarossa G, Attanasio L, Vanelli A, Gasparrini B, Gandolfi F (2010) Culture conditions and signalling networks promoting the establishment of cell lines from parthenogenetic and biparental pig embryos. Stem Cell Rev Rep 6:484–495
Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448:191–195
Cao S, Han J, Wu J, Li Q, Liu S, Zhang W, Pei Y, Ruan X, Liu Z, Wang X, Lim B, Li N (2014) Specific gene-regulation networks during the pre-implantation development of the pig embryo as revealed by deep sequencing. BMC Genomics 15:4
Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S (2005) LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 132:885–896
Cauffman G, De Rycke M, Sermon K, Liebaers I, Van de Velde H (2009) Markers that define stemness in ESC are unable to identify the totipotent cells in human preimplantation embryos. Hum Reprod 24:63–70
Chen LR, Shiue YL, Bertolini L, Medrano JF, BonDurant RH, Anderson GB (1999) Establishment of pluripotent cell lines from porcine preimplantation embryos. Theriogenology 52:195–212
Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh YH, Yeo HC, Yeo ZX, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung WK, Clarke ND, Wei CL, Ng HH (2008) Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133:1106–1117
Dunn SJ, Martello G, Yordanov B, Emmott S, Smith AG (2014) Defining an essential transcription factor program for naive pluripotency. Science 344:1156–1160
Greber B, Wu G, Bernemann C, Joo JY, Han DW, Ko K, Tapia N, Sabour D, Sterneckert J, Tesar P, Scholer HR (2010) Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell 6:215–226
Gu Q, Hao J, Zhao XY, Li W, Liu L, Wang L, Liu ZH, Zhou Q (2012) Rapid conversion of human ESCs into mouse ESC-like pluripotent state by optimizing culture conditions. Protein Cell 3:71–79
Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith A (2009) Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136:1063–1069
Hall VJ, Christensen J, Gao Y, Schmidt MH, Hyttel P (2009) Porcine pluripotency cell signaling develops from the inner cell mass to the epiblast during early development. Dev Dyn 238:2014–2024
Hall VJ, Hyttel P (2014) Breaking down pluripotency in the porcine embryo reveals both a premature and reticent stem cell state in the inner cell mass and unique expression profiles of the naive and primed stem cell states. Stem Cells Dev 23:2030–2045
Haraguchi S, Kikuchi K, Nakai M, Tokunaga T (2012) Establishment of self-renewing porcine embryonic stem cell-like cells by signal inhibition. J Reprod Dev 58:707–716
Hou DR, Jin Y, Nie XW, Zhang ML, Ta N, Zhao LH, Yang N, Chen Y, Wu ZQ, Jiang HB, Li YR, Sun QY, Dai YF, Li RF (2016) Derivation of porcine embryonic stem-like cells from in vitro-produced blastocyst-stage embryos. Sci Rep 6:25838
Kalkan T, Olova N, Roode M, Mulas C, Lee HJ, Nett I, Marks H, Walker R, Stunnenberg HG, Lilley KS, Nichols J, Reik W, Bertone P, Smith A (2017) Tracking the embryonic stem cell transition from ground state pluripotency. Development 144:1221–1234
Kumar RM, Cahan P, Shalek AK, Satija R, DaleyKeyser A, Li H, Zhang J, Pardee K, Gennert D, Trombetta JJ, Ferrante TC, Regev A, Daley GQ, Collins JJ (2014) Deconstructing transcriptional heterogeneity in pluripotent stem cells. Nature 516:56–61
Li M, Ma W, Hou Y, Sun XF, Sun QY, Wang WH (2004) Improved isolation and culture of embryonic stem cells from Chinese miniature pig. J Reprod Dev 50:237–244
Li M, Zhang D, Hou Y, Jiao L, Zheng X, Wang WH (2003) Isolation and culture of embryonic stem cells from porcine blastocysts. Mol Reprod Dev 65:429–434
Liao YJ, Chen YS, Lee JX, Chen LR, Yang JR (2018) Effects of Klf4 and c-Myc knockdown on pluripotency maintenance in porcine induced pluripotent stem cell. Cell J 19:640–646
Lu R, Yang A, Jin Y (2011) Dual functions of T-box 3 (Tbx3) in the control of self-renewal and extraembryonic endoderm differentiation in mouse embryonic stem cells. J Biol Chem 286:8425–8436
Marks H, Kalkan T, Menafra R, Denissov S, Jones K, Hofemeister H, Nichols J, Kranz A, Stewart AF, Smith A, Stunnenberg HG (2012) The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149:590–604
Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113:631–642
Nakamura T, Okamoto I, Sasaki K, Yabuta Y, Iwatani C, Tsuchiya H, Seita Y, Nakamura S, Yamamoto T, Saitou M (2016) A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 537:57–62
Niwa H, Ogawa K, Shimosato D, Adachi K (2009) A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature 460:118–122
Notarianni E, Laurie S, Moor RM, Evans MJ (1990) Maintenance and differentiation in culture of pluripotential embryonic cell lines from pig blastocysts. J Reprod Fertil Suppl 41:51–56
Piedrahita JA, Anderson GB, Bondurant RH (1990) Influence of feeder layer type on the efficiency of isolation of porcine embryo-derived cell lines. Theriogenology 34:865–877
Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK (2008) Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development 135:3081–3091
Scognamiglio R, Cabezas-Wallscheid N, Thier MC, Altamura S, Reyes A, Prendergast AM, Baumgartner D, Carnevalli LS, Atzberger A, Haas S, von Paleske L, Boroviak T, Worsdorfer P, Essers MA, Kloz U, Eisenman RN, Edenhofer F, Bertone P, Huber W, van der Hoeven F, Smith A, Trumpp A (2016) Myc depletion induces a pluripotent dormant state mimicking diapause. Cell 164:668–680
Smith A (2017) Formative pluripotency: the executive phase in a developmental continuum. Development 144:365–373
Strojek RM, Reed MA, Hoover JL, Wagner TE (1990) A method for cultivating morphologically undifferentiated embryonic stem cells from porcine blastocysts. Theriogenology 33:901–913
Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H (2007) Apoptosis and differentiation of human embryonic stem cells induced by sustained activation of c-Myc. Oncogene 26:5564–5576
Tan G, Ren L, Huang Y, Tang X, Zhou Y, Zhou Y, Li D, Song H, Ouyang H, Pang D (2012) Isolation and culture of embryonic stem-like cells from pig nuclear transfer blastocysts of different days. Zygote 20:347–352
Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448:196–199
Theunissen TW, Friedli M, He Y, Planet E, O'Neil RC, Markoulaki S, Pontis J, Wang H, Iouranova A, Imbeault M, Duc J, Cohen MA, Wert KJ, Castanon R, Zhang Z, Huang Y, Nery JR, Drotar J, Lungjangwa T, Trono D, Ecker JR, Jaenisch R (2016) Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell 19:502–515
Turner DA, Trott J, Hayward P, Rue P, Martinez Arias A (2014) An interplay between extracellular signalling and the dynamics of the exit from pluripotency drives cell fate decisions in mouse ES cells. Biol Open 3:614–626
Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, Trotter MW, Cho CH, Martinez A, Rugg-Gunn P, Brons G, Pedersen RA (2009) Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development 136:1339–1349
Varlakhanova NV, Cotterman RF, deVries WN, Morgan J, Donahue LR, Murray S, Knowles BB, Knoepfler PS (2010) myc maintains embryonic stem cell pluripotency and self-renewal. Differentiation 80:9–19
Ying QL, Nichols J, Chambers I, Smith A (2003) BMP induction of id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115:281–292
Zhang J, Ratanasirintrawoot S, Chandrasekaran S, Wu Z, Ficarro SB, Yu C, Ross CA, Cacchiarelli D, Xia Q, Seligson M, Shinoda G, Xie W, Cahan P, Wang L, Ng SC, Tintara S, Trapnell C, Onder T, Loh YH, Mikkelsen T, Sliz P, Teitell MA, Asara JM, Marto JA, Li H, Collins JJ, Daley GQ (2016) LIN28 regulates stem cell metabolism and conversion to primed pluripotency. Cell Stem Cell 19:66–80
Zhang M, Wang C, Jiang H, Liu M, Yang N, Zhao L, Hou D, Jin Y, Chen Q, Chen Y, Wang J, Dai Y, Li R (2019) Derivation of novel naive-like porcine embryonic stem cells by a reprogramming factor-assisted strategy. FASEB J 33:9350–9361
Funding
We received grants from the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine, Jiangsu Province, China. This work was supported by the National Natural Sciences Foundation of China (31371487).
Author information
Authors and Affiliations
Contributions
All authors have contributed to, read, and approved the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editor: Tetsuji Okamoto
Electronic supplementary material
ESM 1
(TIF 896 kb)
Rights and permissions
About this article
Cite this article
Chen, Q., Zhang, H., Jiang, H. et al. Conversion between porcine naïve-like and primed ESCs and specific pluripotency marker identification. In Vitro Cell.Dev.Biol.-Animal 56, 412–423 (2020). https://doi.org/10.1007/s11626-020-00448-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-020-00448-3