Abstract
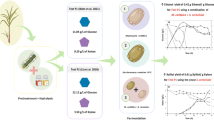
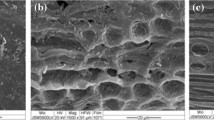
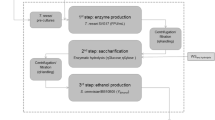
Oleaginous yeast, cultured on second-generation lignocellulosic resources, has the potential to be a key part of the future energy sector. However, the multiple unit operations necessary to produce concentrated hydrolysates, with a minimum of fermentation inhibitors, limit the applicability to date. In this study, a simple microwave-assisted hydrothermal pre-treatment step of oak or beech sawdust was deployed to produce an oligosaccharide-rich hydrolysate. This was then catabolised by the oleaginous yeast, Metschnikowia pulcherrima, avoiding the need for costly enzymatic or further chemical steps in the processing. Up to 85% of the sawdust’s hemicelluloses could be solubilised under these conditions, and 8 g/L DCW yeast with a 42% lipid content produced. While a number of studies have demonstrated that oleaginous yeasts possess high inhibitor tolerance, using this real lignocellulosic hydrolysate, we demonstrate that lipid production is actually very sensitive to inhibitor and carbon availability, and the optimal system is not the one that gives the highest hydrolysate or cell biomass. Indeed, the yeast was shown to detoxify the inhibitors in the process, but at high inhibitor loading, this leads to very poor lipid production, especially at high furfural levels. These findings clearly highlight the importance of considering multiple variables when real, complex lignocellulosic media are involved, tuning process conditions based on the desired fermentation outcomes.






Similar content being viewed by others
Abbreviations
- OPEX:
-
Operating expenses
- M. pulcherrima :
-
Metschnikowia pulcherrima
- 5-HMF:
-
5-Hydroxymethylfurfural
- Furf:
-
Furfural
- 2-PE:
-
2-Phenylethanol
- DPs:
-
Degree of polymerisation
- XMG:
-
Sum of xylose, mannose and galactose
- DCW:
-
Dry cell weight
- SEM:
-
Standard error of the means
- SCC:
-
Shewart Control Charts
- OOC:
-
Out of control
- UCL:
-
Upper control line
- LCL:
-
Lower control line
- SD:
-
Standard deviation
- μ max:
-
Maximum growth rate
References
Singhvi MS, Chaudhari S, Gokhale DV (2014) Lignocellulose processing: a current challenge. RSC Adv 4(16):8271–8277. https://doi.org/10.1039/C3RA46112B
Gregg D, Boussaid A, Saddler J (1998) Techno-economic evaluations of a generic wood-to-ethanol process: effect of increased cellulose yields and enzyme recycle. Bioresour Technol 63(1):7–12
Remón J, Santomauro F, Chuck CJ, Matharu AS, Clark JH (2018) Production of fermentable species by microwave-assisted hydrothermal treatment of biomass carbohydrates: reactivity and fermentability assessments. Green Chem 20(19):4507–4520. https://doi.org/10.1039/c8gc02182a
Adhikari S, Ozarska B (2018) Minimizing environmental impacts of timber products through the production process From Sawmill to Final Products. Environmental Systems Research 7(1):6. https://doi.org/10.1186/s40068-018-0109-x
Puligundla P, Oh S-E, Mok C (2016) Microwave-assisted pretreatment technologies for the conversion of lignocellulosic biomass to sugars and ethanol: a review. Carbon Letters (Carbon Lett) 17(1):1–10. https://doi.org/10.5714/CL.2016.17.1.001
Fan J, De Bruyn M, Budarin VL, Gronnow MJ, Shuttleworth PS, Breeden S, Macquarrie DJ, Clark JH (2013) Direct microwave-assisted hydrothermal depolymerization of cellulose. J Am Chem Soc 135(32):11728–11731. https://doi.org/10.1021/ja4056273
Fan J, Santomauro F, Budarin VL, Whiffin F, Abeln F, Chantasuban T, Gore-Lloyd D, Henk D, Scott RJ, Clark J (2018) The additive free microwave hydrolysis of lignocellulosic biomass for fermentation to high value products. J Clean Prod 198:776–784. https://doi.org/10.1016/j.jclepro.2018.07.088
Santomauro F, Whiffin FM, Scott RJ, Chuck CJ (2014) Correction: low-cost lipid production by an oleaginous yeast cultured in non-sterile conditions using model waste resources. Biotechnology for Biofuels 7(1):42. https://doi.org/10.1186/1754-6834-7-42
Abeln F, Fan J, Budarin VL, Briers H, Parsons S, Allen MJ, Henk DA, Clark J, Chuck CJ (2019) Lipid production through the single-step microwave hydrolysis of macroalgae using the oleaginous yeast Metschnikowia pulcherrima. Algal Res 38:101411. https://doi.org/10.1016/j.algal.2019.101411
Barbosa C, Lage P, Esteves M, Chambel L, Mendes-Faia A, Mendes-Ferreira A (2018) Molecular and phenotypic characterization of Metschnikowia pulcherrima strains from Douro Wine Region. Fermentation 4(1):8. https://doi.org/10.3390/fermentation4010008
Oro L, Ciani M, Comitini F (2014) Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J Appl Microbiol 116(5):1209–1217. https://doi.org/10.1111/jam.12446
Sitepu I, Selby T, Lin T, Zhu S, Boundy-Mills K (2014) Carbon source utilization and inhibitor tolerance of 45 oleaginous yeast species. J Ind Microbiol Biotechnol 41(7):1061–1070. https://doi.org/10.1007/s10295-014-1447-y
Gore-Lloyd D, Sumann I, Brachmann AO, Schneeberger K, Ortiz-Merino RA, Moreno-Beltran M, Schlafli M, Kirner P, Santos Kron A, Rueda-Mejia MP, Somerville V, Wolfe KH, Piel J, Ahrens CH, Henk D, Freimoser FM (2019) Snf2 controls pulcherriminic acid biosynthesis and antifungal activity of the biocontrol yeast Metschnikowia pulcherrima. Mol Microbiol 112(1):317–332. https://doi.org/10.1111/mmi.14272
Krause DJ, Kominek J, Opulente DA, Shen XX, Zhou X, Langdon QK, DeVirgilio J, Hulfachor AB, Kurtzman CP, Rokas A, Hittinger CT (2018) Functional and evolutionary characterization of a secondary metabolite gene cluster in budding yeasts. Proc Natl Acad Sci 115(43):11030–11035. https://doi.org/10.1073/pnas.1806268115
Sipiczki M (2003) Candida zemplinina sp. nov., an osmotolerant and psychrotolerant yeast that ferments sweet botrytized wines. Int J Syst Evol Microbiol 53(6):2079–2083. https://doi.org/10.1099/ijs.0.02649-0
Türkel S, Korukluoğlu M, Yavuz M (2014) Biocontrol activity of the local strain of Metschnikowia pulcherrima on different postharvest pathogens. Biotechnol Res Int 2014:1–6
Strauss MLA, Jolly NP, Lambrechts MG, van Rensburg P (2001) Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J Appl Microbiol 91(1):182–190. https://doi.org/10.1046/j.1365-2672.2001.01379.x
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure 1617:1–16
Abeln F, Chuck CJ (2019) Achieving a high-density oleaginous yeast culture: comparison of four processing strategies using Metschnikowia pulcherrima. Biotechnol Bioeng 116(12):3200–3214. https://doi.org/10.1002/bit.27141
Pan L-X, Yang D-F, Shao L, Li W, Chen G-G, Liang Z-Q (2009) Isolation of the oleaginous yeasts from the soil and studies of their lipid-producing capacities. Food Technol Biotechnol 47(2):215–220
Anhøj J, Wentzel-Larsen T (2018) Sense and sensibility: on the diagnostic value of control chart rules for detection of shifts in time series data. BMC Med Res Methodol 18(1):100. https://doi.org/10.1186/s12874-018-0564-0
Yoon HH (1998) Pretreatment of lignocellulosic biomass by autohydrolysis and aqueous ammonia percolation. Korean J Chem Eng 15(6):631–636. https://doi.org/10.1007/BF02698990
Zhu P, Tang Y, Q-s XUE, J-f LI, Lu Y (2009) Microwave-assisted hydrolysis of cellulose using metal chloride as Lewis acid catalysts. J Fuel Chem Technol 2:244–247
Poerschmann J, Weiner B, Koehler R, Kopinke F-D (2017) Hydrothermal carbonization of glucose, fructose, and xylose identification of organic products with medium molecular masses. ACS Sustain Chem Eng 5(8):6420–6428
Leschinsky M, Sixta H, Patt R (2009) Detailed mass balances of the autohydrolysis of Eucalyptus globulus at 170 C. BioResources 4(2):687–703
Jiang Z, Fan J, Budarin VL, Macquarrie DJ, Gao Y, Li T, Hu C, Clark JH (2018) Mechanistic understanding of salt-assisted autocatalytic hydrolysis of cellulose. Sustainable Energy & Fuels 2(5):936–940
Yang M, Huo R, Shen H, Xia Q, Qiu J, Robertson AW, Li X, Sun Z (2020) Metal-tuned W18O49 for efficient electrocatalytic N2 reduction. ACS Sustain Chem Eng 8(7):2957–2963
Nitsos CK, Choli-Papadopoulou T, Matis KA, Triantafyllidis KS (2016) Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis. ACS Sustain Chem Eng 4(9):4529–4544. https://doi.org/10.1021/acssuschemeng.6b00535
Zhou L, Santomauro F, Fan J, Macquarrie D, Clark J, Chuck CJ, Budarin V (2017) Fast microwave-assisted acidolysis: a new biorefinery approach for the zero-waste utilisation of lignocellulosic biomass to produce high quality lignin and fermentable saccharides. Faraday Discuss 202:351–370
Chen X, Li Z, Zhang X, Hu F, Ryu DD, Bao J (2009) Screening of oleaginous yeast strains tolerant to lignocellulose degradation compounds. Appl Biochem Biotechnol 159(3):591–604. https://doi.org/10.1007/s12010-008-8491-x
van der Pol EC, Vaessen E, Weusthuis RA, Eggink G (2016) Identifying inhibitory effects of lignocellulosic by-products on growth of lactic acid producing micro-organisms using a rapid small-scale screening method. Bioresour Technol 209:297–304. https://doi.org/10.1016/j.biortech.2016.03.037
Aggelis G, Komaitis M, Papanikolaou S, Papadopoulos G (1995) A mathematical model for the study of lipid accumulation in oleaginous microorganisms. I. Lipid accumulation during growth of Mucor circinelloides CBS 172-27 on a vegetable oil. GRASAS Y ACEITES-SEVILLA 46:169–169. https://doi.org/10.3989/gya.1995.v46.i3.921
Papanikolaou S, Aggelis G (2003) Modeling lipid accumulation and degradation in Yarrowia lipolytica cultivated on industrial fats. Curr Microbiol 46(6):398–402. https://doi.org/10.1007/s00284-002-3907-2
Economou CN, Aggelis G, Pavlou S, Vayenas DV (2011) Modeling of single-cell oil production under nitrogen-limited and substrate inhibition conditions. Biotechnol Bioeng 108(5):1049–1055. https://doi.org/10.1002/bit.23026
Almeida JRM, Modig T, Petersson A, Hähn-Hägerdal B, Lidén G, Gorwa-Grauslund MF (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol 82(4):340–349. https://doi.org/10.1002/jctb.1676
Acknowledgements
The authors thank Dr. Hannah Briers and Paul Elliott for expert technical assistance.
Funding
This work is financially supported by the Industrial Biotechnology Catalyst (Innovate UK, BBSRC, EPSRC) to support the translation, development and commercialisation of innovative industrial Biotechnology processes (EP/N013522/1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 9095 kb)
Rights and permissions
About this article
Cite this article
Longanesi, L., Bouxin, F.P., Fan, J. et al. Valorisation of sawdust through the combined microwave-assisted hydrothermal pre-treatment and fermentation using an oleaginous yeast. Biomass Conv. Bioref. 12, 2487–2499 (2022). https://doi.org/10.1007/s13399-020-00757-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00757-3