Abstract
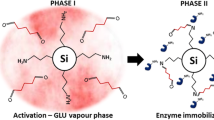
In this study, biodegradation of tetracycline, which is one of the most commonly used antibiotics, was investigated. In order to convert tetracycline into inactive oxidative metabolites, laccase immobilized onto rigid tripolyphosphate-treated chitosan beads was utilized. For immobilization, chitosan surfaces were activated with glutaraldehyde-linker reagents after tripolyphosphate treatment. The glutaraldehyde-linked rigid chitosan beads showed the higher immobilization capacity of laccase, providing thermostability, reusability, and the higher tetracycline transformation activity. Subsequently, synthetic mediators, including 2,2'‑azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) and lignophenolic mediators like violuric, syringic and ferulic acids, 1-hydroxybenzotriazole, and vanillin, were investigated to activate laccase as electron mediators with KI assistance. Among them, the immobilized laccase showed highest tetracycline antibiotic inactivation activity when ABTS was used as a mediator. In the presence of KI, violuric and ferulic acids, 1-hydroxybenzotriazole and vanillin functioned as laccase mediators and inactivated tetracycline almost completely, however, the effect was not observed when they did individually. Finally, Vmax of the immobilized laccase was determined as 76.3 ± 4.3 µmoles mg–1 min–1 against tetracycline, which was 2-fold higher than that of the free enzyme. These results will provide novel insights into laccase-based removal of non-biodegradable organic matter.





Similar content being viewed by others
REFERENCES
Liu, X., Steele, J.C., and Meng, X.Z., Environ. Pollut., 2017, vol. 223, pp. 161–169.
Rajapakse, S., Chrishan Shivanthan, M., Samaranayake, N., and Rodrigo, C., Deepika Fernando, S., Pathog. Glob. Health, 2013, vol. 107, no. 4, pp. 162–169.
Kullar, R., Sakoulas, G., Deresinski, S., and van Hal, S. J., J. Antimicrob. Chemother., 2016, vol. 71, no. 3, pp. 576–586.
Arda, B., Yamazhan, T., Sipahi, O.R., Islekel, S., Buke, C., and Ulusoy, S., Int. J. Antimicrob. Agents, 2005, vol. 25, no. 5, pp. 414–418.
Huo, T.I., J. Chin. Med. Assoc., 2010, vol. 73, no. 11, pp. 557–558.
Van Boeckel, T.P., Gandra, S., Ashok, A., Caudron, Q., Grenfell B.T., Levin S.A., and Laxminarayan R., Lancet. Infect. Dis., 2014, vol. 14, no. 8, pp. 742–750.
Klein, E.Y., Van Boeckel, T.P., Martinez, E.M., Pant, S., Gandra, S., Levin, S. A., et al., Proc. Natl. Acad. Sci. U. S. A., 2018, vol. 115, no. 15, pp. E3463–E3470.
Cai, M., Ma, S., Hu, R., Tomberlin, J.K., Yu, C., Huang, Y., et al., Environ. Pollut., 2018, vol. 242, pt. A, pp. 634–642.
Chen, J., Michel, F.C., Jr., Sreevatsan, S., Morrison, M., and Yu, Z., Microb. Ecol., 2010, vol. 60, no. 3, pp. 479–486.
Laht, M., Karkman, A., Voolaid, V., Ritz, C., Tenson, T., Virta, M., and Kisand, V., PLoS One, 2014, vol. 9, no. 8. e103705.
Kim, S., Eichhorn, P., Jensen, J.N., Weber, A.S., and Aga, D.S., Environ. Sci. Technol., 2005, vol. 39, no. 15, pp. 5816–5823.
Miyata, M., Ihara, I., Yoshid, G., Toyod, K., and Umetsu, K., Water Sci. Technol., 2011, vol. 63, no. 3, pp. 456–461.
de Godos, I., Munoz, R., and Guieysse, B., J. Hazard. Mater., 2012, vol. 229–230, pp. 446–449.
Liu, L., Liu, Y.H., Wang, Z., Liu, C.X., Huang, X., and Zhu, G.F., J. Hazard. Mater., 2014, vol. 278, pp. 304–310.
Wen, X., Jia, Y., and Li, J., J. Hazard. Mater., 2010, vol. 177, no. 1–3, pp. 924–928.
Wen, X., Jia, Y., and Li, J., Chemosphere, 2009, vol. 75, no. 8, pp. 1003–1007.
Sun, K., Huang, Q., and Li, S., J. Hazard. Mater., 2017, vol. 331, pp. 182–188.
Yang, J., Lin, Y., Yang, X., Ng, T.B., Ye, X., and Lin, J., J. Hazard. Mater., 2017, vol. 322, no. Pt B, pp. 525–531.
Ding, H., Wu, Y., Zou, B., Lou, Q., Zhang, W., Zhong, J., et al., J. Hazard. Mater., 2016, vol. 307, pp. 350–358.
Kudanga, T., Nyanhongo, G.S., Guebitz, G.M., and Burton, S., Enzyme Microb. Technol., 2011, vol. 48, no. 3, pp. 195–208.
Morozova, O.V., Shumakovich, G.P., Shleev, S.V., and Iaropolov, A.I., Appl. Biochem. Microbiol., 2007, vol. 43, no. 5, pp. 583–597.
Asgher, M., Noreen, S., and Bilal, M., Int. J. Biol. Macromol., 2017, vol. 95, pp. 54–62.
Huber, D., Tegl, G., Baumann, M., Sommer, E., Gorji, E. G., Borth, N., et al., Carbohydr. Polym., 2017, vol. 157, pp. 814–822.
Shi, H., Peng, J., Li, J., Mao, L., Wang, Z., and Gao, S., J. Hazard. Mater., 2016, vol. 317, pp. 81–89.
Kadam, A. A., and Lee, D. S., Bioresour. Technol., 2015, vol. 193, pp. 563–567.
Poon, L., Wilson, L. D., and Headley, J. V., Carbohydr. Polym., 2014, vol. 109, pp. 92–101.
Rocasalbas, G., Francesko, A., Tourino, S., Fernandez-Francos, X., Guebitz, G. M., and Tzanov, T., Carbohydr. Polym., 2013, vol. 92, no. 2, pp. 989–996.
Gabriel Paulraj, M., Ignacimuthu, S., Gandhi, M.R., Shajahan, A., Ganesan, P., Packiam, S.M., and Al-Dhabi, N.A., Int. J. Biol. Macromol., 2017, vol. 104, no. Pt B, pp. 1813–1819.
Buranachai, T., Praphairaksit, N., and Muangsin, N., AAPS Pharm. Sci.Tech., 2010, vol. 11, no. 3, pp. 1128–1137.
Xu, H. and Matysiak, S., Chem. Commun. (Camb.)., 2017, vol. 53, no. 53, pp. 7373–7376.
Hilgers, R., Vincken, J. P., Gruppen, H., and Kabel, M. A., ACS Sustain. Chem. Eng., 2018, vol. 6, no. 2, pp. 2037–2046.
Shah, M. M. and Aust, S. D., J. Biol. Chem., 1993, vol. 268, no. 12, pp. 8503–8506.
Boschloo, G. and Hagfeldt, A., Acc. Chem. Res., 2009, vol. 42, no. 11, pp. 1819–1826.
Kulys, J., Krikstopaitis, K., and Ziemys, A., J. Biol. Inorg. Chem., 2000, vol. 5, no. 3, pp. 333–340.
Funding
This work was supported by the Ajou University research fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Jeong, D., Choi, KY. Biodegradation of Tetracycline Antibiotic by Laccase Biocatalyst Immobilized on Chitosan-Tripolyphosphate Beads. Appl Biochem Microbiol 56, 306–312 (2020). https://doi.org/10.1134/S0003683820030047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683820030047