Abstract
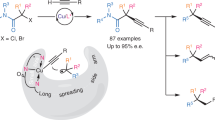
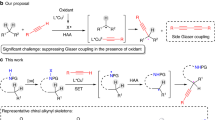
In contrast to the wealth of enantioselective prochiral C(sp3)–H functionalization that is transition-metal-catalysed, the enantioconvergent transformation of racemic tertiary C(sp3)–H bonds (pKa > 25) still represents a vastly uncharted domain. The mechanistic limitation is partial or complete chirality retention, which is inherent to developed enantioselective C–H functionalization catalysis and poses the major challenge in establishing such a process. To this end, we herein describe the combination of decoupled hydrogen atom abstraction with asymmetric copper catalysis for enantioconvergent tertiary β-C(sp3)–H amination of racemic ketones. This method, when allied with follow-up transformations, provides facile access to a range of enantioenriched compounds featuring quaternary stereocentres. We anticipate that this work will inspire the future design of generally efficient catalysts for enantioconvergent transformations of racemic tertiary C(sp3)–H bonds.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The findings of this study are available within the paper and its Supplementary Information. Crystallographic parameters for compounds 14 and 62 are available free of charge from the Cambridge Crystallographic Data Centre under CCDC 1923926 (14) and 1923927 (62). All data are available from the authors upon reasonable request.
References
You, S.-L. (ed.) Asymmetric Functionalization of C–H Bonds (The Royal Society of Chemistry, 2015).
Newton, C. G., Wang, S.-G., Oliveira, C. C. & Cramer, N. Catalytic enantioselective transformations involving C–H bond cleavage by transition-metal complexes. Chem. Rev. 117, 8908–8976 (2017).
Saint-Denis, T. G., Zhu, R.-Y., Chen, G., Wu, Q.-F. & Yu, J.-Q. Enantioselective C(sp 3)‒H bond activation by chiral transition metal catalysts. Science 359, eaao4798 (2018).
Davies, H. M. L. & Manning, J. R. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008).
Che, C.-M., Lo, V. K.-Y., Zhou, C.-Y. & Huang, J.-S. Selective functionalisation of saturated C–H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 40, 1950–1975 (2011).
Roizen, J. L., Harvey, M. E. & du Bois, J. Metal-catalyzed nitrogen-atom transfer methods for the oxidation of aliphatic C–H bonds. Acc. Chem. Res. 45, 911–922 (2012).
Davies, H. M. L. & Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 3, 347–360 (2019).
Lu, H. & Zhang, X. P. Catalytic C–H functionalization by metalloporphyrins: recent developments and future directions. Chem. Soc. Rev. 40, 1899–1909 (2011).
Lewis, J. C., Coelho, P. S. & Arnold, F. H. Enzymatic functionalization of carbon–hydrogen bonds. Chem. Soc. Rev. 40, 2003–2021 (2011).
Milan, M., Bietti, M. & Costas, M. Enantioselective aliphatic C–H bond oxidation catalyzed by bioinspired complexes. Chem. Commun. 54, 9559–9570 (2018).
Wang, Y., Wen, X., Cui, X. & Zhang, X. P. Enantioselective radical cyclization for construction of 5-membered ring structures by metalloradical C–H alkylation. J. Am. Chem. Soc. 140, 4792–4796 (2018).
Bhat, V., Welin, E. R., Guo, X. & Stoltz, B. M. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations. Chem. Rev. 117, 4528–4561 (2017).
Choi, J. & Fu, G. C. Transition metal–catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C–N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Wendlandt, A. E., Vangal, P. & Jacobsen, E. N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature 556, 447–451 (2018).
Wang, Z., Yin, H. & Fu, G. C. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature 563, 379–383 (2018).
Zhang, X. et al. An enantioconvergent halogenophilic nucleophilic substitution (SN2X) reaction. Science 363, 400–404 (2019).
Yang, Y., Cho, I., Qi, X., Liu, P. & Arnold, F. H. An enzymatic platform for the asymmetric amination of primary, secondary and tertiary C(sp 3)–H bonds. Nat. Chem. 11, 987–993 (2019).
Paradine, S. M. et al. A manganese catalyst for highly reactive yet chemoselective intramolecular C(sp 3)–H amination. Nat. Chem. 7, 987–994 (2015).
Barton, D. H. R., Beaton, J. M., Geller, L. E. & Pechet, M. M. A new photochemical reaction. J. Am. Chem. Soc. 83, 4076–4083 (1961).
Hofmann, A. W. Ueber die einwirkung des broms in alkalischer lösung auf die amine. Ber. Dtsch. Chem. Ges. 16, 558–560 (1883).
Löffler, K. & Freytag, C. Über eine neue bildungsweise von N-alkylierten pyrrolidinen. Ber. Dtsch. Chem. Ges. 42, 3427–3431 (1909).
Sharma, A. & Hartwig, J. F. Metal-catalysed azidation of tertiary C–H bonds suitable for late-stage functionalization. Nature 517, 600–604 (2015).
Mukherjee, S., Maji, B., Tlahuext-Aca, A. & Glorius, F. Visible-light-promoted activation of unactivated C(sp 3)–H bonds and their selective trifluoromethylthiolation. J. Am. Chem. Soc. 138, 16200–16203 (2016).
Liu, Z. et al. Copper‐catalyzed remote C(sp 3)−H trifluoromethylation of carboxamides and sulfonamides. Angew. Chem. Int. Ed. 58, 2510–2513 (2019).
Salamone, M. & Bietti, M. Tuning reactivity and selectivity in hydrogen atom transfer from aliphatic C–H bonds to alkoxyl radicals: role of structural and medium effects. Acc. Chem. Res. 48, 2895–2903 (2015).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 115, 9587–9652 (2015).
Fu, G. C. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 3, 692–700 (2017).
Gu, Q.-S., Li, Z.-L. & Liu, X.-Y. Copper(i)-catalyzed asymmetric reactions involving radicals. Acc. Chem. Res. 53, 170–181 (2020).
Dong, X.-Y. et al. A general asymmetric copper-catalysed Sonogashira C(sp 3)–C(sp) coupling. Nat. Chem. 11, 1158–1166 (2019).
Zhang, W. et al. Enantioselective cyanation of benzylic C–H bonds via copper-catalyzed radical relay. Science 353, 1014–1018 (2016).
Li, J. et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature 574, 516–521 (2019).
Gao, D.-W., Zheng, J., Ye, K.-Y., Zheng, C. & You, S.-L. in Asymmetric Functionalization of C–H Bonds 199–202 (The Royal Society of Chemistry, 2015).
Ma, J. et al. Visible-light-activated asymmetric β-C–H functionalization of acceptor-substituted ketones with 1,2-dicarbonyl compounds. J. Am. Chem. Soc. 139, 17245–17248 (2017).
Rottmann, M. et al. Spiroindolones, a potent compound class for the treatment of malaria. Science 329, 1175–1180 (2010).
Wang, X. et al. Aromatic spiroketal bisphosphine ligands: palladium-catalyzed asymmetric allylic amination of racemic Morita–Baylis–Hillman adducts. Angew. Chem. Int. Ed. 51, 9276–9282 (2012).
Ji, X. & Huang, H. Synthetic methods for 1,3-diamines. Org. Biomol. Chem. 14, 10557–10566 (2016).
Salaün, J. in Small Ring Compounds in Organic Synthesis VI 1–67 (Springer, 2000).
Dalpozzo, R., Lattanzi, A. & Pellissier, H. Applications of chiral three-membered rings for total synthesis: a review. Curr. Org. Chem. 21, 1143–1191 (2017).
Cvrtila, I., Fanlo-Virgós, H., Schaeffer, G., Monreal Santiago, G. & Otto, S. Redox control over acyl hydrazone photoswitches. J. Am. Chem. Soc. 139, 12459–12465 (2017).
Duan, X.-Y., Yang, X.-L., Jia, P.-P., Zhang, M. & Han, B. Hydrazonyl radical-participated tandem reaction: a strategy for the synthesis of pyrazoline-functionalized oxindoles. Org. Lett. 17, 6022–6025 (2015).
Acknowledgements
Financial support for this work was provided by the National Natural Science Foundation of China (nos. 21722203, 21702093, 21831002 and 21801116), Guangdong Provincial Key Laboratory of Catalysis (no. 2020B121201002), Guangdong Innovative Program (no. 2019BT02Y335), Guangdong Basic and Applied Basic Research Foundation (no. 2019A1515110822), Shenzhen Special Funds (nos. JCYJ20170412152435366, JCYJ20180302180235837 and JCYJ20180302174416591), Shenzhen Nobel Prize Scientists Laboratory Project (no. C17783101), and SUSTech Special Fund for the Construction of High-Level Universities (no. G02216303).
Author information
Authors and Affiliations
Contributions
C.-J.Y., C.Z. and Q.-S.G. designed the experiments and analysed the data. C.-J.Y., C.Z., J.-H.F., X.-L.S., L.Y., Y.S., Y.T. and Z.-L.L. performed the experiments. All authors participated in writing the manuscript. X.-Y.L. conceived and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4, Tables 1–4, Methods, NMR spectra, HPLC spectra and references.
Crystallographic Data 1
Crystallographic data for compound 14
Crystallographic Data 2
Crystallographic data for compound 62
Rights and permissions
About this article
Cite this article
Yang, CJ., Zhang, C., Gu, QS. et al. Cu-catalysed intramolecular radical enantioconvergent tertiary β-C(sp3)–H amination of racemic ketones. Nat Catal 3, 539–546 (2020). https://doi.org/10.1038/s41929-020-0460-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0460-y
This article is cited by
-
Construction of chiral through-space luminophores via symmetry breaking triggered by sequenced chlorination
Science China Chemistry (2023)
-
Photoelectrochemical asymmetric catalysis enables site- and enantioselective cyanation of benzylic C–H bonds
Nature Catalysis (2022)
-
Asymmetric benzylic C(sp3)−H acylation via dual nickel and photoredox catalysis
Nature Communications (2021)
-
Catalytic enantioselective C(sp3)–H functionalization involving radical intermediates
Nature Communications (2021)