Abstract
Purpose
To collate evidence of changes in body composition following treatment of leukaemia in children, teenagers and young adults (CTYA, 0–24 years) with allogeneic haematopoietic stem cell transplant and total body irradiation (HSCT+TBI).
Methods
Papers were identified by searching Medline and Google Scholar, reference lists/citations and contacting key authors, with no date or language restrictions. Inclusion criteria were as follows: leukaemia, HSCT+TBI, aged ≤ 24 years at HSCT and changes in body composition (total fat, central adiposity, adipose tissue function, muscle mass, muscle function). Quality was assessed using a brief Newcastle–Ottawa scale.
Results
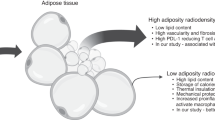
Of 900 papers, 20 were included: seven controlled, five uncontrolled studies and eight case reports. Study quality appeared good. There was little evidence of differences in total fat/weight for HSCT + TBI groups (compared to healthy controls/population norms/short stature controls). There was some evidence of significantly higher central adiposity and differences in adipose tissue function (compared to leukaemic/non-leukaemic controls). Muscle mass was significantly lower (compared to healthy/obese controls). Muscle function results were inconclusive but suggested impairment. Case reports confirmed a lipodystrophic phenotype.
Conclusions
Early remodelling of adipose tissue and loss of skeletal muscle are evident following HSCT + TBI for CTYA leukaemia, with extreme phenotype of overt lipodystrophy. There is some evidence for reduced muscle effectiveness.
Implications for Cancer Survivors
Body composition changes in patients after HSCT + TBI are apparent by early adult life and link with the risk of excess cardiometabolic morbidity seen in adult survivors. Interventions to improve muscle and/or adipose function, perhaps utilizing nutritional manipulation and/or targeted activity, should be investigated.
Similar content being viewed by others

Introduction
Leukaemia is the commonest type of cancer in children (0 to < 16 years) and one of the most common diagnoses affecting teenagers and young adults (16 to < 25 years). Patients who fail primary treatment, or those with very high risk factors at diagnosis, may be treated with allogeneic haematopoietic stem cell transplantation (HSCT) after conditioning with high dose chemotherapy and total body irradiation (TBI) [1]. Adult survivors of HSCT with TBI conditioning experience long-term morbidity, impaired quality of life and reduced life expectancy. Endocrine disorders including growth hormone deficiency, hypothyroidism and gonadal failure are well-described, but there is now good evidence of a phenotype emerging in early adult life that resembles accelerated ageing [2] with early post-transplant telomere shortening [3], long-term metabolic dysfunction [4], abnormal body composition [5], frailty [6] and fatigue [7]. Investigation has identified specific findings which incorporate features of the metabolic syndrome including hypertension, dyslipidaemia, insulin resistance, visceral adiposity and a pro-inflammatory state [8, 9].
Screening for adverse adiposity that increases cardiometabolic risk in the general population is relatively easy using standard measures of obesity (raised BMI and/or waist circumference) but is less straightforward in HSCT/TBI survivors who may not be overtly obese by these criteria. In contrast, the phenotype is characterized by the presence of increased visceral but reduced subcutaneous fat and reduced lean mass, i.e. they also demonstrate, at extremes, overt sarcopenic and lipodystrophic phenotypes [10]. These changes seem causally linked to the increased risk of metabolic syndrome in this patient population [1]. Metabolic syndrome has six components that relate to cardiovascular disease risk (based on the ATPIII definition): abdominal obesity, atherogenic dyslipidaemia, raised blood pressure, insulin resistance ± glucose intolerance, proinflammatory and prothrombotic states [11].
Survivors of all forms of cancer diagnosed as children or as teenagers and young adults (CTYA), including leukaemia treated without HSCT/TBI, may also face long-term morbidity in adult life depending on the nature of the treatment received; cardiovascular disease is the most common cause of early mortality in CTYA cancer survivors after the risk of death from second cancer [12]. Metabolic syndrome is also reported in other survivors of childhood cancer, but HSCT, TBI and cranial or abdominal irradiation all appear to incur greater risk [13]. Recent studies also confirm an increased risk of type 2 diabetes in adult survivors of childhood leukaemia [14].
The incidence, severity, progression and outcome of changes in body composition/BMI in survivors of HSCT/TBI undertaken in the CTYA age range are unclear. Nor is it known how their risk compares with survivors of CTYA leukaemia treated without HSCT/TBI or with individuals without a history of cancer treatment with or without evidence of obesity. Clarifying the phenotype of HSCT/TBI survivors may assist in developing future studies to investigate the critical pathophysiological changes that drive the associated cardiometabolic consequences likely to occur in adult life and in designing potential interventions.
Aims
This restricted systematic review aimed to:
-
collate evidence of changes in body composition/BMI in survivors of leukaemia treated in the CTYA age range (age 0–< 25 years) with HSCT with TBI
-
identify evidence that body composition is associated with change in metabolic status in survivors
-
describe dietary and exercise interventions used to ameliorate these changes in body composition
-
compare findings, with studies of leukaemia survivors treated without HSCT with TBI and with the general population
Methods
This review was registered on PROSPERO International prospective register of systematic reviews, reference number CRD42019138493. We followed Plüddemann’s framework for rapid reviews [15].
Searches
Papers were identified by:
-
Searching Medline via OVID using Medical Subject Headings (MeSH) and keyword terms (see Online Resource 1), with weekly email updates for papers published since the search. Medline was searched from its inception to the date of search (May 2019)
-
Searching Google Scholar (first 20 pages of results) using search terms in Online Resource 1
-
Contacting key authors (lead authors on included papers) to identify any work-in-progress or unpublished work
-
Checking reference lists of and citations to key articles
Study selection
Titles and abstracts were assessed for eligibility by AL, with RP independently assessing a random sample of 10% of records. Articles meeting inclusion criteria were retrieved in full and independently considered by two reviewers (MS, JHS). The reviewers resolved disagreements through discussion; reasons for excluding studies were recorded in a table.
The inclusion criteria were:
-
Participants—we included studies of people:
-
Treated for all types of leukaemia with the addition of cases of non-Hodgkin’s lymphoma (NHL) and myelodysplastic syndrome (MDS) if included within a study of leukaemia patients
-
Treated with allogeneic HSCT and TBI (or both allogeneic and autologous if the allogeneic participants are analysed separately)
-
Aged up to and including 24 years (i.e. to 25th birthday) at HSCT
-
Any age at the time of evaluation
-
Studies including multiple conditions if leukaemia patients made up ≥ 90% of the sample or if results for leukaemia were analysed separately. Also, studies including patients treated with and without TBI if those with TBI made up ≥ 90% of the sample or results were analysed for TBI vs no TBI
-
-
Comparators
-
Studies with or without a comparator
-
-
Characteristics—studies which measured body composition changes, any of:
-
Sarcopenia (including impaired muscle strength)
-
Frailty (self-reported exhaustion, weakness (grip strength), slow walking speed, low physical activity and unintentional weight loss [16]
-
Lipodystrophy (abnormal fat distribution)
-
Changes in fat distribution, e.g. increased visceral/central fat
-
Changes in fat compartmentation/positioning
-
Body mass index (BMI)
-
-
Intervention studies must use the intervention after the HSCT not before.
-
Study design
-
Completed studies
-
With or without control groups
-
With or without interventions
-
Including case studies, feasibility studies, cohort studies
-
Literature reviews were only included in order to identify primary studies in their reference lists.
-
No date or language restrictions were applied.
Non-English papers were translated where possible.
Data extraction
Full-text articles for inclusion were retrieved, and data extracted using a standardized data extraction template by AL, with RP independently extracting data from a random sample of 20% of articles and JHS and MS each independently extracting from a random sample of 10% of articles. Data extracted included the following: study methods (aim, setting, sample eligibility criteria, data collection methods and timing), participant flow (numbers eligible/recruited/followed up, reasons for non-participation), participant characteristics (diagnoses, treatment details, age at HSCT, age at follow up, sex, ethnicity) and outcome data (for each outcome, subgroup comparisons). The primary outcome data collected were:
-
Total fat, e.g. BMI, whole body % fat
-
Central adiposity, e.g. waist circumference, abdominal fat
-
Adipose tissue function, e.g. adipokines, lipids
-
Muscle mass, e.g. sarcopenia, frailty, lean body mass, fat-free mass
-
Muscle function, e.g. muscle strength tests, frailty.
Secondary outcomes, only collected if body composition changes were also described:
-
Measures of insulin resistance, glucose tolerance and metabolic syndrome
For studies which used interventions, we ensured adverse event data was extracted. The template was piloted before starting the review and modified as required to ensure consistency. Disagreements in opinion of data extracted were resolved through discussion.
Quality assessment
To assess the quality of included studies, AL used a modified, brief Newcastle–Ottawa quality assessment scale [17]. Quality scores are reported in a table.
Results
Search results
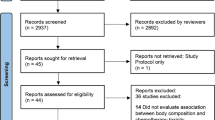
Figure 1 details the search process. A total of 900 papers were identified, of which 880 were excluded. The most common reasons for exclusion were that studies were not about cancer or had no body composition outcomes (full reasons are given in Fig. 1). Of 24 emails to key authors, we received nine responses.
A final total of 20 papers were included—seven controlled studies [1, 10, 18,19,20,21,22], five uncontrolled studies [23,24,25,26,27] and eight case reports/series [28,29,30,31,32,33,34,35]. Only one study included an intervention [23].
Our exclusion criteria aimed to create a homogeneous set of papers relevant to a future study of interventions for body composition and frailty in childhood leukaemia HSCT with TBI. However, we are aware that some of the excluded papers may include relevant information so have provided these references in Online Resource 2.
Study designs
Table 1 gives details of the twelve controlled and uncontrolled studies and outcome data are summarized in Table 2. ALL was the most common diagnostic group. Four studies included a range of diagnoses. Four of the seven controlled studies had two control groups and three studies only one. Controls included leukaemia patients without HSCT (5 studies), healthy people (3 studies) and other clinical groups (short stature or obese; 3 studies). Studies were conducted between 6 and 16.7 years after HSCT. Of our chosen body composition outcomes, eleven of the twelve studies measured total body fat, seven measured central adiposity and six measured adipose tissue function. Only four measured muscle mass and muscle function.
The eight case reports/series are presented in Table 3, representing a total of eleven cases.
Study quality
The assessment of study quality was brief (using a modified Newcastle–Ottawa scale with 8 very simple criteria). As shown in Table 4, apart from a lack of blinding of outcome assessors (not present in any study), most studies fulfilled most criteria.
Outcome data
Table 2 provides the outcome data for the controlled and uncontrolled studies. Outcomes for the case reports are included in Table 3. Due to heterogeneity within included studies (especially in terms of outcomes), we did not synthesize the data or perform any meta-analysis.
Changes in body composition
The body composition results of the studies are reported in Table 2 and briefly summarized below.
Total body fat
There was little evidence of differences in total fat/weight between HSCT + TBI groups and healthy controls, population norms or short stature controls. Nysom et al. found significantly lower BMI compared to healthy controls [20]. Wei et al. also found significantly lower BMI and fat mass index, but this was compared to obese controls [10, 22]. Three studies found significantly higher body fat: body fat % compared to short stature controls [19] and healthy controls [20] and whole body fat mass z score compared to reference controls [1]. Data from Adachi et al. [26] suggests BMI may be lower than leukaemic controls with no TBI, and, although significance could not be tested, within the normal range for age.
Central adiposity
Most of the studies which measured central adiposity found significantly higher central adiposity for HSCT + TBI groups compared to leukaemic controls and non-leukaemic (obese/short stature/healthy) controls. Evidence from four studies found significant differences for lower waist-to-hip ratios and higher android-gynoid fat ratios compared to leukaemic controls and for higher waist circumference/waist–height ratio, greater trunk fat % and visceral fat %, compared to non-obese non-leukaemic controls [1, 19, 22, 118]. One study found evidence of significant differences for lower waist circumference/waist–height ratio, higher visceral fat % and higher visceral fat to total/subcutaneous fat ratios compared to obese non-leukaemic controls [10].
High waist-to-hip ratio was associated with features of metabolic syndrome in one study [22], and visceral fat % was associated with insulin resistance in another [1].
Adipose tissue function
All three studies which measured adipose tissue function found significant differences for HSCT + TBI groups compared to leukaemic controls and some to non-leukaemic controls. Compared to leukaemic controls, adiponectin was lower, leptin higher, triglycerides higher and high-density lipoprotein (HDL) lower [10, 18, 22]. The only difference compared to non-leukaemic controls (obese) was for raised triglycerides [22]. Lower adiponectin and HDL levels were more common in those with insulin resistance [1, 18].
Muscle mass
Four studies measured muscle mass (fat free/lean mass, muscle density), and all found significantly lower muscle mass for HSCT + TBI groups compared to healthy/obese controls and in HSCT + TBI patients compared with findings before HSCT + TBI [1, 10, 19, 27]. Wei et al. [10] found limited evidence for lower fat-free mass index compared to leukaemic controls. Lean mass/height2 was lower in females [27].
Muscle function
For HSCT + TBI groups compared to leukaemic controls, Taskinen et al. [21] found significant differences in some physical performance tests but not others, and Chow et al. found lower physical activity scores [18]. Davis et al. found some increase in strength following an exercise intervention [23].
Association of body composition changes with metabolic status
Some studies commented on associations of body composition outcomes with the presence of features of metabolic syndrome. Associations with metabolic syndrome/insulin resistance were found with:
-
Whole body fat mass [1]
-
Waist-to-hip ratio and waist-to-height ratio [22]
-
Subcutaneous adipose tissue, visceral adipose tissue [1]
-
Lower adiponectin levels [25]
-
Lower HDL [25]
Potential factors modifying impact of HSCT on body composition
Although not an aim of this review, most studies reported on certain factors which may impact the relationship between HSCT and body composition, in particular graft versus host disease (GVHD), growth hormone deficiency and cranial radiation. This section briefly reports these results.
GVHD and treatment
Most studies reported the number of participants with GVHD, which varied from 0 to 61.5%. However, there was wide variability in reporting this and the details, i.e. whether acute or chronic. This is not a primary focus for this review. One study found that GVHD was predictive of underweight post-HSCT, and extensive chronic GVHD was predictive of lower BMI, but this was an uncontrolled study [27]. Three studies reported that GVHD or glucocorticoid treatment was not associated with body composition (cytokine levels [18]; marrow adipose tissue, any measures of adiposity or lean mass [1]; or whole-body % fat z score [20]).
GH
Two studies found an association of GH status with fat mass index (FMI) [10, 19] and gynoid fat% [10], but not with fat-free mass index (FFMI) [19], and other studies found no associations with body composition (cytokine levels [18], adiponectin [10], central fat [10] or different fat deposits from magnetic resonance imaging [10]).
Cranial radiation
Some studies explored the association of cranial radiation with body composition and found differences in BMI and whole body fat [20] but not in cytokine levels [18] or cardiometabolic traits [18].
Age at/time since HSCT
The studies showed mixed results regarding the relationship between time since HSCT and body composition. Age at HSCT was not associated with body composition in two studies (adiposity or lean mass [1] or whole-body % fat z score [20]).Two studies found no association (components of the metabolic syndrome [22], whole-body % fat z score [20] or measures of adiposity [1]) but did find a negative association with HDL [22] and adiponectin levels [10].
Interventions to ameliorate changes in body composition
Only one study included an intervention [23]. The intervention (a 6-month programme of supervised, combined resistance and aerobic exercise) significantly improved aerobic fitness, insulin resistance and quality of life, although there were no changes in body composition. The authors concluded that the intervention had a metabolic training effect on muscle.
Case reports
Table 3 presents characteristics and body composition data from the eleven cases reported in the eight case reports/series [28,29,30,31,32,33,34,35]. Seven had ALL and four AML; ten were female and one male. The cases were followed up an average of 11 years after HSCT. Nine of the eleven cases had GVHD and most had multiple complications/other diagnoses.
The data reported in the case reports/series presents a phenotype of lipodystrophy in leukaemic HSCT TBI patients which appears well described. All the cases were under- or normal weight based on their BMI (range 12.2 to 23.1) but showed clinical evidence of lipodystrophy with reduced fat in the limbs and gluteal region and increased fat centrally and in the face, with abdominal distension. Dyslipidaemia was noted in many cases, with elevated fasting triglycerides of between 332 and 927 mg/dL (3.75–10.5 mmol/L) (normal range < 150 mg/dL or < 1.7 mmol/L) but seemingly normal leptin levels of 3.5–7.4 ng/mL (normal range for females 8.8 + SEM 2.10 ng/mL [36]). Only one case report mentioned muscle function (limited range of motion and poor muscle tone); none of the reports mentioned muscle mass changes.
Discussion
This review has found evidence that following HSCT with TBI as treatment for leukaemia in CTYA before the age of 25 years, there is remodelling of adipose tissue earlier than would be expected for age and an extreme phenotype of overt lipodystrophy. There is also some evidence for frailty and a reduction in muscle effectiveness/bulk/strength. These changes are associated with evidence for metabolic disadvantage which contributes to the risk of cardiovascular disease, particularly as abdominal obesity has been shown to be a risk factor for insulin resistance and impaired glucose tolerance following HSCT [37]. Although the literature is heterogeneous, limiting the conclusions we can draw, other studies of wider populations (not just leukaemia or not all TBI; excluded from our review) confirm this phenotype—for example reduced lean mass/increased fat mass for height in HSCT patients [5], increased abdominal adiposity and hypertriglyceridemia [38] and increased sarcopenia [39].
Although the mechanisms for how HSCT with TBI affects body composition was a not a focus for this review, some studies mentioned factors which may additionally impact on body composition, including GVHD, growth hormone deficiency and cranial radiation. There is a need to understand why the changes in muscle and fat occur following HSCT.
Clinical implications
The 2012 guidelines on screening and preventive practices for long-term survivors of HSCT [40] include recommendations for early treatment of cardiovascular risk factors such as diabetes, hypertension and dyslipidaemia and education and counselling on healthy lifestyle (regular exercise, maintaining healthy weight, no smoking, dietary counselling). Griffith et al. [41] also provide detailed recommendations on the evaluation and management of dyslipidaemia in HSCT patients. Nevertheless, the key issue is whether any interventions can be shown to help mitigate or even reverse the adverse changes to body composition and the apparent link to the cardiometabolic risk.
We only identified one study which tested an intervention [23]; whilst this showed effects on fitness, insulin resistance and quality of life, it did not demonstrate any effects on body composition. Studies on wider populations have found some positive effects for exercise and nutritional supplementation during or after TBI: increased body mass and BMI, partly mediated by an increase in fat-free mass [42]; improved muscular strength and endurance performance [43]; increased fat free mass and decreased body fat [44]; and improved muscle mass [45].
Many conventional weight loss techniques would not be appropriate in this population as patients after HSCT with TBI are not overweight and nonspecific weight loss could exacerbate their situation due to further loss of muscle mass. Although one study demonstrated the feasibility and acceptability of a strength-training intervention for patients undergoing HSCT [46], it is possible that exercise benefits may be limited, due to reduced muscle mass. There is therefore a need to develop innovative interventions to improve the muscle function and metabolic effectiveness of long-term survivors of HSCT with TBI in the CTYA years, perhaps utilizing dietary supplements and targeted forms of physical activity.
Limitations
There are limitations to this review. As a restricted systematic review, the screening of articles was less comprehensive than for a systematic review and there is a chance that eligible papers were excluded. We have included in Online Resource 2 lists of excluded papers. Responses from key authors in the field confirmed that we had identified most relevant studies. Searching only one database may have meant we missed relevant papers. However, this methodology is acceptable for a restricted systematic review, and we also attempted to identify grey literature and did not limit by date or language [15].
This review did not aim to identify potential mechanisms leading to body composition changes, so did not systematically collect data on associations with factors such as GVHD, additional/prior radiotherapy, e.g. to the central nervous system or abdomen, or endocrine status.
Most of the included studies were not designed with body composition as their primary outcome, meaning our final sample covered a very diverse range of study designs and outcomes, making data synthesis difficult. The variation in demographics of the study populations makes it difficult to compare outcome data to population norms.
The studies included also have their own limitations. Studies all used convenience samples, with very little information reported on those who did not volunteer to participate. We are therefore unable to comment on how representative our results are to the general leukaemia HSCT with TBI population. Few studies reported participants’ ethnicity or were mostly composed of those with white ethnicities, which is a potential deficiency given that ethnicity can affect body composition and the risk of metabolic disruption when abnormal [47].
Conclusion
This review has found evidence that allogeneic HSCT with TBI for CTYA leukaemia results in remodelling of adipose tissue earlier than is expected for age, with the extreme phenotype of overt lipodystrophy. There is also some evidence for a reduction in muscle effectiveness/bulk/strength. These changes mirror those seen with normal ageing and appear to associate with measures of early cardiovascular morbidity. Innovative interventions are needed to determine if changes in muscle and adipose function and metabolic effectiveness can be reversed/mitigated at any age after HSCT, perhaps utilizing dietary manipulation and/or targeted exercise and activity interventions.
References
Mostoufi-Moab S, Magland J, Isaacoff EJ, Sun W, Rajapakse CS, Zemel B, et al. Adverse fat depots and marrow adiposity are associated with skeletal deficits and insulin resistance in long-term survivors of pediatric hematopoietic stem cell transplantation. J Bone Miner Res. 2015;30(9):1657–66.
Skinner R, von Zglinicki T. Accelerated aging in bone marrow transplant survivors accelerated aging in bone marrow transplant survivors editorial. JAMA Oncol. 2016;2(10):1267–8. https://doi.org/10.1001/jamaoncol.2016.0877.
Rufer N, Brümmendorf TH, Chapuis B, Helg C, Lansdorp PM, Roosnek E. Accelerated telomere shortening in hematological lineages is limited to the first year following stem cell transplantation. Blood. 2001;97(2):575–7. https://doi.org/10.1182/blood.V97.2.575.
Oudin C, Auquier P, Bertrand Y, Contet A, Kanold J, Sirvent N, et al. Metabolic syndrome in adults who received hematopoietic stem cell transplantation for acute childhood leukemia: an LEA study. Bone Marrow Transplant. 2015;50(11):1438–44.
Mostoufi-Moab S, Ginsberg JP, Bunin N, Zemel BS, Shults J, Thayu M, et al. Body composition abnormalities in long-term survivors of pediatric hematopoietic stem cell transplantation. J Pediatr. 2012;160(1):122–8.
Arora M, Sun CL, Ness KK, Teh JB, Wu J, Francisco L, et al. Physiologic frailty in nonelderly hematopoietic cell transplantation patients: results from the Bone Marrow Transplant Survivor Study. JAMA Oncol. 2016;2(10):1277–86.
Tomlinson D, Baggott C, Dix D, Gibson P, Hyslop S, Johnston DL, et al. Severely bothersome fatigue in children and adolescents with cancer and hematopoietic stem cell transplant recipients. Support Care Cancer. 2018;27:2665–71. https://doi.org/10.1007/s00520-018-4555-9.
Inamoto Y, Lee SJ. Late effects of blood and marrow transplantation. Haematologica. 2017;102(4):614–25. https://doi.org/10.3324/haematol.2016.150250.
Bielorai B, Pinhas-Hamiel O. Type 2 diabetes mellitus, the metabolic syndrome, and its components in adult survivors of acute lymphoblastic leukemia and hematopoietic stem cell transplantations. Curr Diab Rep. 2018;18(6):32. https://doi.org/10.1007/s11892-018-0998-0.
Wei C, Thyagiarajan MS, Hunt LP, Shield JP, Stevens MC, Crowne EC. Reduced insulin sensitivity in childhood survivors of haematopoietic stem cell transplantation is associated with lipodystropic and sarcopenic phenotypes. Pediatr Blood Cancer. 2015;62(11):1992–9.
Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–7. https://doi.org/10.1242/dmm.001180.
Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304(2):172–9. https://doi.org/10.1001/jama.2010.923.
Pluimakers VG, van Waas M, Neggers S, van den Heuvel-Eibrink MM. Metabolic syndrome as cardiovascular risk factor in childhood cancer survivors. Crit Rev Oncol Hematol. 2019;133:129–41. https://doi.org/10.1016/j.critrevonc.2018.10.010.
Williams HE, Howell CR, Chemaitilly W, Wilson CL, Karol SE, Nolan VG, et al. Diabetes mellitus among adult survivors oc childhood acute lymphoblastic leukemia: a report from the St Jude Lifetime Cohort Study. Cancer. 2019. https://doi.org/10.1002/cncr.32596.
Plüddemann A, Aronson JK, Onakpoya I, Heneghan C, Mahtani KR. Redefining rapid reviews: a flexible framework for restricted systematic reviews. BMJ Evid Based Med. 2018;23(6):201–3. https://doi.org/10.1136/bmjebm-2018-110990.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. https://doi.org/10.1093/gerona/56.3.m146.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Chow EJ, Simmons JH, Roth CL, Baker KS, Hoffmeister PA, Sanders JE, et al. Increased cardiometabolic traits in pediatric survivors of acute lymphoblastic leukemia treated with total body irradiation. Biol Blood Marrow Transplant. 2010;16(12):1674–81.
Davis NL, Stewart CE, Moss AD, Woltersdorf WW, Hunt LP, Elson RA, et al. Growth hormone deficiency after childhood bone marrow transplantation with total body irradiation: interaction with adiposity and age. Clin Endocrinol. 2015;83(4):508–17.
Nysom K, Holm K, Michaelsen KF, Hertz H, Jacobsen N, Muller J, et al. Degree of fatness after allogeneic BMT for childhood leukaemia or lymphoma. Bone Marrow Transplant. 2001;27(8):817–20.
Taskinen MH, Kurimo M, Kanerva J, Hovi L. Physical performance of nontransplanted childhood ALL survivors is comparable to healthy controls. J Pediatr Hematol Oncol. 2013;35(4):276–80.
Wei C, Hunt L, Cox R, Bradley K, Elson R, Shield J, et al. Identifying cardiovascular risk in survivors of childhood leukaemia treated with haematopoietic stem cell transplantation and total body irradiation. Horm Res Paediatr. 2017;87(2):116–22.
Davis NL, Tolfrey K, Jenney ME, Elson R, Stewart CB, Moss AD et al. Combined resistance and aerobic exercise intervention improves fitness, insulin resistance, and quality of life (QoL) in survivors of childhood haemopoietic stem cell transplantation (HSCT) with total body irradiation (TBI). IN PREPARATION. ND.
Freycon F, Trombert-Paviot B, Casagranda L, Mialou V, Berlier P, Berger C, et al. Final height and body mass index after fractionated total body irradiation and allogeneic stem cell transplantation in childhood leukemia. Pediatr Hematol Oncol. 2012;29(4):313–21.
Chemaitilly W, Boulad F, Oeffinger KC, Sklar CA. Disorders of glucose homeostasis in young adults treated with total body irradiation during childhood: a pilot study. Bone Marrow Transplant. 2009;44(6):339–43.
Adachi M, Oto Y, Muroya K, Hanakawa J, Asakura Y, Goto H. Partial lipodystrophy in patients who have undergone hematopoietic stem cell transplantation during childhood: an institutional cross-sectional survey. Clin Pediatr Endocrinol. 2017;26(2):99–108.
Inaba H, Yang J, Kaste SC, Hartford CM, Motosue MS, Chemaitilly W, et al. Longitudinal changes in body mass and composition in survivors of childhood hematologic malignancies after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2012;30(32):3991–7.
Ceccarini G, Ferrari F, Santini F. Acquired partial lipodystrophy after bone marrow transplant during childhood: a novel syndrome to be added to the disease classification list. J Endocrinol Investig. 2017;40(11):1273–4.
Kimura L, Alvarez G, Li N, Pawlikowska-Haddal A, Moore TB, Casillas J, et al. Temporary resolution of insulin requirement in acquired partial lipodystrophy associated with chronic graft-versus-host disease. Pediatr Blood Cancer. 2017;64(7).
Rajendran R, Abu E, Fadl A, Byrne CD. Late effects of childhood cancer treatment: severe hypertriglyceridaemia, central obesity, non alcoholic fatty liver disease and diabetes as complications of childhood total body irradiation. Diabet Med. 2013;30(8):e239–e42. https://doi.org/10.1111/dme.12234.
Rooney DP, Ryan MF. Diabetes with partial lipodystrophy following sclerodermatous chronic graft vs. host disease. Diabet Med. 2006;23(4):436–40.
Adachi M, Asakura Y, Muroya K, Goto H, Kigasawa H. Abnormal adipose tissue distribution with unfavorable metabolic profile in five children following hematopoietic stem cell transplantation: a new etiology for acquired partial lipodystrophy. Clin Pediatr Endocrinol. 2013;22(4):53–64.
Hosokawa M, Shibata H, Hosokawa T, Irie J, Ito H, Hasegawa T. Acquired partial lipodystrophy with metabolic disease in children following hematopoietic stem cell transplantation: a report of two cases and a review of the literature. J Pediatr Endocrinol Metab. 2019;32(5):537. https://doi.org/10.1515/jpem-2018-0356.
Mayson SE, Parker VE, Schutta MH, Semple RK, Rickels MR. Severe insulin resistance and hypertriglyceridemia after childhood total body irradiation. Endocr Pract. 2013;19(1):51–8.
Amin P, Shah S, Walker D, Page SR. Adverse metabolic and cardiovascular risk following treatment of acute lymphoblastic leukaemia in childhood; two case reports and a literature review. Diabet Med. 2001;18(10):849–53. https://doi.org/10.1046/j.1464-5491.2001.00591.x.
Al-Sultan AI, Al-Elq AH. Leptin levels in normal weight and obese saudi adults. J Fam Community Med. 2006;13(3):97–102.
Neville KA, Cohn RJ, Steinbeck KS, Johnston K, Walker JL. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J Clin Endocrinol Metab. 2006;91(11):4401–7.
Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356(9234):993–7. https://doi.org/10.1016/s0140-6736(00)02717-3.
Jabbour J, Manana B, Zahreddine A, Saade C, Charafeddine M, Bazarbachi A, et al. Sarcopenic obesity derived from PET/CT predicts mortality in lymphoma patients undergoing hematopoietic stem cell transplantation. Curr Res Transl Med. 2019;67(3):93–9. https://doi.org/10.1016/j.retram.2018.12.001.
Majhail NS, Douglas Rizzo J, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Hematol/Oncol Stem Cell Ther. 2012;5(1):1–30. https://doi.org/10.5144/1658-3876.2012.1.
Griffith ML, Savani BN, Boord JB. Dyslipidemia after allogeneic hematopoietic stem cell transplantation: evaluation and management. Blood. 2010;116(8):1197–204. https://doi.org/10.1182/blood-2010-03-276576.
Chamorro-Vina C, Ruiz JR, Santana-Sosa E, Gonzalez Vicent M, Madero L, Perez M, et al. Exercise during hematopoietic stem cell transplant hospitalization in children. Med Sci Sports Exerc. 2010;42(6):1045–53.
Baumann FT, Zopf EM, Nykamp E, Kraut L, Schüle K, Elter T, et al. Physical activity for patients undergoing an allogeneic hematopoietic stem cell transplantation: benefits of a moderate exercise intervention. Eur J Haematol. 2011;87(2):148–56. https://doi.org/10.1111/j.1600-0609.2011.01640.x.
Hayes S, Davies PS, Parker T, Bashford J. Total energy expenditure and body composition changes following peripheral blood stem cell transplantation and participation in an exercise programme. Bone Marrow Transplant. 2003;31(5):331–8.
Ren G, Zhang J, Li M, Yi S, Xie J, Zhang H, et al. Protein blend ingestion before allogeneic stem cell transplantation improves protein-energy malnutrition in patients with leukemia. Nutr Res. 2017;46:68–77.
Hacker ED, Larson JL, Peace D. Exercise in patients receiving hematopoietic stem cell transplantation: lessons learned and results from a feasibility study. Oncol Nurs Forum. 2011;38(2):216–23.
Maffeis C, Morandi A. Body composition and insulin resistance in children. Eur J Clin Nutr. 2018;72(9):1239–45. https://doi.org/10.1038/s41430-018-0239-2.
Acknowledgements
We are very grateful to Dr. Alyson Huntley for her expert advice on literature reviewing, and also the authors who responded to our requests for information (Claudio Annaloro, Eric Chow, Masanori Adachi, Giovanni Ceccarini, Nikki Davis, Charles Sklar, Sogol Mostoufi-Moab, Guangxu Ren, and Christina Wei). We are particularly grateful to Dr. Adachi for sharing his original data.
CTYA HSCT Adipose and Muscle late effects working group: Stephen Wootton and Martin Feelisch (University of Southampton), Lars O. Dragsted (University of Copenhagen, Denmark), Marlou Dirks (University of Exeter), Saeed Shoaie and Adil Mardinoglu (Kings College, London), Helen Roche (University College Dublin, Ireland), Julian Hamilton-Shield and Michael Stevens (University of Bristol).
Funding
This study was funded by a Wellcome Trust Nutrition Award (grant number 215859/Z/19/Z). JHS and RP are supported, and this work was conducted within an NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclaimer
The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lorenc, A., Hamilton-Shield, J., Perry, R. et al. Body composition after allogeneic haematopoietic cell transplantation/total body irradiation in children and young people: a restricted systematic review. J Cancer Surviv 14, 624–642 (2020). https://doi.org/10.1007/s11764-020-00871-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-020-00871-1