Abstract
Purpose
To analyze photoreceptors’ condition after intravitreal ranibizumab treatment according to the pattern of diabetic macular edema (DME) on spectral-domain optical coherence tomography (SD-OCT).
Methods
Retrospective study includes 58 treatment naïve patients with DME, treated with intravitreal ranibizumab injections and followed up for at least 12 months. Patients were classified based on DME morphology on SD-OCT into: diffuse macular edema, cystoid macular edema (CME) and serous retinal detachment with CME (SRD/CME). The DME morphology was analyzed, while quantitative measurement of ellipsoid zone (EZ) defect, as well as qualitative assessment of the condition of external limiting membrane (ELM) and interdigitation zone (IZ) at foveal area, was taken before and after treatment.
Results
Before treatment, patients with CME presented worse ELM and IZ condition and greater EZ defect than patients with diffuse macular edema or SRD/CME. After treatment, the restoration of EZ defect and IZ was more evident in patients with CME than in diffuse macular edema or SRD/CME.
Conclusion
Patients with DME presented significant photoreceptors’ restoration after intravitreal ranibizumab injections at the 12-month follow-up. The improvement in EZ defect size and IZ was dependent on the pattern of DME on SD-OCT.
Similar content being viewed by others
Introduction
Diabetic macular edema (DME) is the leading cause of visual impairment in patients with diabetes mellitus, occurring in approximately 35% of patients with diabetic retinopathy (DR) [1, 2]. In the pathogenesis of DME, hyperglycemia promotes structural changes in retinal blood vessels and disruption of the blood–retinal barrier, resulting in accumulation of fluid [3]. Besides the vasogenic theory, there is some degree of neuronal death implicated in DR [4] and photoreceptors’ involvement, leading to their neurodegeneration [5]. Otani et al. have described three major patterns of DME based on optical coherence tomography (OCT); sponge-like swelling, cystoid macular edema (CME) and serous retinal detachment (SRD) associated with CME [6]. Nowadays, the advent of spectral-domain OCT (SD-OCT), besides the description of the morphological pattern of DME, has also facilitated the detailed study of the status of retinal photoreceptors, allowing identification of the external limiting membrane (ELM), the photoreceptor ellipsoid zone (EZ) and the interdigitation zone (IZ) [7].
Vascular endothelial growth factor (VEGF) is the most potent angiogenic factor and a prominent mediator of vascular permeability in patients with DME [8]. Therefore, the gold standard in the treatment of DME is anti-VEGF agents, whose safety and efficacy have been proven in large pivotal clinical trials, as well as in real-life studies [9, 10]. Only a few studies have examined whether the response to anti-VEGF treatment changes according to the morphological type of DME [11,12,13,14]. Diffuse macular edema has showed the greatest improvement in central retinal thickness and visual acuity after intravitreal bevacizumab [11, 12], while Seo et al. found no difference in treatment response between DME types using intravitreal ranibizumab [13]. In addition, a significant restoration of photoreceptors after intravitreal ranibizumab treatment, correlating with visual acuity, has been reported [15]. The purpose of the current study is to analyze the photoreceptors’ changes after anti-VEGF treatment, stratifying patients according to the DME pattern on SD-OCT.
Methods
In this retrospective study, the medical records of 58 adult patients (58 eyes) with type 2 diabetes mellitus and treatment naïve DME (Table 1), who were diagnosed and treated at 2nd Department of Ophthalmology, University of Athens, Greece, between January 2016 and June 2018, were reviewed and analyzed. All patients received intravitreal ranibizumab injections and were followed-up for at least 12 months. In case where both eyes of the same patient presented DME, we included only one eye per patient. Specifically, the right eyes of patients with bilateral DME were selected, so as to avoid selection bias and intercorrelation of measurements in the same patient. Patients with other retinal diseases, vitreomacular traction, macular hole, proliferative diabetic retinopathy, ocular inflammation, myopia > 6D, uncontrolled glaucoma, previous vitrectomy, ocular trauma or cataract surgery within the last 6 months and media opacities were excluded from the study. The study was in accordance with the Tenets of Helsinki Declaration, and written informed consent was obtained by all patients to use their data.
Data related to demographic characteristics, medical history, smoking status, comorbidities (hypertension, hyperlipidemia, heart failure, nephropathy) and HbA1c were recorded for all patients. All participants had underwent a complete ophthalmological examination at the time of DME diagnosis (baseline), including best-corrected visual acuity (BCVA) measurement using Snellen charts, biomicroscopy, dilated fundoscopy, spectral-domain optical coherence tomography (SD-OCT) and fluorescein angiography (FFA) using Spectralis (Spectralis HRA + OCT, Heidelberg Engineering, Germany).
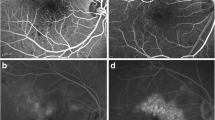
For each patient, BCVA was converted to the logarithm of the minimum angle of resolution (logMAR) for statistical purposes. Regarding SD-OCT examination, six radial scans 3-mm long were performed at equally spaced angular orientations centered on the foveola. An OCT volume scan was performed on a 20° × 20° cube, consisting of 49 horizontal B-scans with 20 averaged frames per B-scan centered over the fovea, each containing 1064 pixels, separated by 125 mm. OCT scans were evaluated for macular thickness, which was automatically measured at central area of ETDRS map, the type of DME (diffuse, CME and SRD combined with CME, as it is shown in Fig. 1), the location of DME (predominantly in inner or outer layers), the extent of DME (involving the fovea or extending outside the fovea) and the presence of epiretinal membrane (ERM-defined as a thin, hyperreflective band on the surface of the retina above the internal limiting membrane). The quantitative measurement of EZ defect size at fovea was taken manually using the OCT-caliper of the SD-OCT machine by two trained investigators (IP and ED). The structural condition of ELM and IZ at the fovea was categorized qualitatively as intact (if it was continuous and completely visible), or disrupted (if it was disrupted, attenuated because of pathological changes or absent) by the same trained investigators (IP, ED). If reliable evaluation of the three zones (ELM, EZ and IZ) could not be performed due to poor image quality or due to DME, the patient was excluded from the study.
Patterns of diabetic macular edema in spectral-domain optical coherence tomography: a diffuse macular edema; b cystoid macular edema; c serous retinal detachment combined with cystoid macular edema. Please note external limiting membrane (orange arrow), ellipsoid zone (green arrow) and interdigitation zone (blue arrow)
All patients were treated with at least three monthly intravitreal ranibizumab injections as a loading dose. Thereafter, all patients were followed-up at a pro re nata (PRN) basis, with monthly monitoring for at least 12 months. At each monthly visit, all patients underwent BCVA measurement and SD-OCT. Re-injection was performed if the height of macular edema was ≥ 320 μm and BCVA decreased ≥ 1 Snellen line.
Statistical analysis was performed using SPSS 22.0 statistical software (SPSS Inc, Chicago, Illinois, USA). Descriptive statistics, including the mean values, median, standard deviations and percentages, were used to describe the baseline characteristics. Differences between the three groups were evaluated with one-way analysis of variance (ANOVA) for numerical data and with Chi-square test for categorical data. For longitudinal comparisons, the Wilcoxon signed-rank test was performed for numerical data and McNemar’s test for categorical data. Since multiple comparisons took place (baseline vs. month 6 or 12), a Bonferroni correction was adopted, as appropriate. A p value less than 0.05 was defined as statistically significant.
Results
Table 1 shows the demographic and clinical characteristics of the study sample at baseline.
Sub-analysis based on the patterns of DME on SD-OCT is shown in Table 2. Patients having CME had a larger EZ defect size (467 ± 103 μm) compared to those with diffuse macular edema (346 ± 102 μm) and SRD/CME (286 ± 100 μm), (p < 0.001). In cases with diffuse macular edema, the EZ defect extended mainly outside the fovea. Patients with CME presented disruption in IZ in a higher percentage than patients with diffuse macular edema or SRD/CME (p < 0.001), while ELM was significantly more intact in patients with SRD/CME (p < 0.001).
In the whole cohort, there was a statistically significant improvement in BCVA (0.27 ± 0.09 logMAR, p < 0.001) and in CRT (368.0 ± 99.3 μm, p < 0.001) at month 12 post-treatment. The evolution of BCVA and CRT over time in the whole cohort and in the three groups of DME separately showed greater improvement in CME group (Figs. 2 and 3). The number of injections at the end of the follow-up period of 12 months was 7.4 ± 1.3 in patients with CME, 6.1 ± 1.2 in patients with SRD/CME and 5.9 ± 1.3 in patients with diffuse macular edema (p = 0.036).
The EZ defect size improved significantly at month 12 post-treatment (459 ± 123 at baseline vs. 293 ± 109 at month 12, p < 0.001). Only patients with CME and SRD/CME showed significant difference in EZ defect size at month 12 compared to baseline. Specifically, patients with CME demonstrated greater improvement in the EZ defect size (467 ± 103 μm at baseline vs. 312 ± 102 μm at month 12, p < 0.001), compared to patients with SRD/CME (286 ± 100 μm at baseline vs. 205 ± 101 μm at month 12, p < 0.001) and patients with diffuse macular edema (346 ± 102 μm at baseline vs. 301 ± 113 at month 12, p = 0.102).
There was a statistically significant restoration of IZ at month 6 (69.0% intact IZ at baseline vs. 77.6% at month 6, p = 0.038) and month 12 post-treatment (69.0% intact IZ at baseline vs. 86.2% at month 12, p < 0.001). The restoration in IZ was more prominent in cases with CME (61.3% intact IZ at baseline vs. 87.1% at month 12, p < 0.001). However, in patients with diffuse macular edema, the IZ showed restoration which did not reach statistical significance (76.2% intact IZ at baseline vs. 85.7% at month 12, p = 0.068). In patients with SRD/CME, the condition of IZ remained stable (83.3% intact IZ at both time-points).
Discussion
The principal message of this study is that the various DME patterns on SD-OCT present different response after treatment with intravitreal ranibizumab injections, with significant difference in the outer retinal layers. At baseline patients with diffuse macular edema had better photoreceptors’ condition, BCVA and lower CRT than those with CME. EZ size defect was greater in patients with CME, since macular edema was mainly located at the fovea and the outer retinal layers, compared to patients with diffuse macular edema, in whom macular edema extended outside the fovea and was located mainly in the inner retinal layers. Additionally, IZ was more affected in patients with CME than with diffuse macular edema. BCVA improvement and the CRT reduction were observed in the whole cohort, as well as in each group. Controversy exists about how anti-VEGF treatment affects the neuroglial dysfunction in DME and contributes to the photoreceptors’ restoration [5, 13, 15].
In the current study, we measured manually the EZ defect size and found that there was significant improvement in EZ defect size post-intravitreal ranibizumab injections. Significant improvement of EZ defect size was observed in patients with CME and SRD/CME, while patients with diffuse macular edema showed slight improvement that did not reach significance. Regarding the IZ restoration, significant restoration was observed in the whole cohort at months 6 and 12 after intravitreal ranibizumab injections. Patients with CME presented significant IZ restoration at the end of the follow-up of 12 months, while IZ condition did not differ significantly in patients with diffuse macular edema and SRD/CME.
The EZ represents the photoreceptor integrity and is mainly comprised of mitochondria, enabling higher levels of energy consumption in the photoreceptors, while IZ is believed to represent RPE microvilli that surround the cone outer segment terminals [16]. In patients with DME, it is hypothesized that the mitochondrial dysfunction in the foveal photoreceptors results in reduced VA. Cystoid spaces located in the foveal area may compress and deform the photoreceptors [17], as it has been shown in our study in patients with CME. On the other hand, in our study, patients with diffuse macular edema did not present significant restoration in EZ, but only a slight improvement or stabilization of the EZ condition. It seems that the resolution of cysts after anti-VEGF therapy may result in photoreceptors’ layer improvement. As far as IZ is concerned, we found that IZ improvement occurred after EZ recovery, as it was observed previously by Serizawa et al. [18]. The IZ condition was significantly improved in patients with CME, but not in those with diffuse macular edema, in line with the EZ defect size improvement, which was statistically significant in patients with CME and not in patients with diffuse macular edema.
The ELM represents the junctional complex between the Mueller cells and the photoreceptor cells and has barrier properties against macromolecules [19]. The disruption of the ELM might allow blood constituents to pour into the subretinal space and damage the photoreceptors, or vice versa, photoreceptor damage might lead to disruption of the ELM in DME [20]. In our study, restoration of EZ and IZ at the end of the follow-up was correlated with ELM condition and in eyes where ELM was absent no restoration of EZ or IZ was observed. This has been previously described in patients with successful macular hole closure and optic disk pit maculopathy post-treatment, showing that the restoration of EZ is directly related to the integrity of the ELM and to the extent of EZ defect before treatment [21,22,23]. Ito et al. have also reported that ELM, EZ and IZ condition are all positively correlated with VA in patients with DME [24], as it was found in our study as well.
Even though Ramon Y Cajal proposed that when neurons degenerate, they do not regenerate [25], other publications have reported neuronal regeneration in several situations [26]. In patients with DME, it could not be determined whether the restoration of EZ/IZ on the SD-OCT corresponds to regeneration of the photoreceptor cells [13]. Spaide et al. have described that in some eyes with DME, although the ELM and the EZ were disrupted, outer nuclear layer exists just above these lesions [16]. This might explain why restoration of EZ may be observed after anti-VEGF treatment.
Potential limitation of the study pertains to the fact that marked destruction of the retinal layers occurs in the majority of DME cases. Moreover, shadowing artifacts could theoretically be responsible for variable response in different DME patterns. In addition, the EZ defect measurement has been performed manually using the OCT-caliper. It has also to be mentioned that macular edema can fluctuate significantly, depending on intraobserver, interobserver and diurnal variation. However, to our knowledge this is the first study, which examined the impact of various patterns of DME in SD-OCT to the photoreceptors’ response after treatment with intravitreal ranibizumab injections.
In conclusion, this study described the morphological differences on SD-OCT between the three types of DME and was concentrated on photoreceptors’ condition before and after intravitreal ranibizumab treatment, based on different DME patterns on SD-OCT. Intravitreal anti-VEGF injections may lead to regeneration of photoreceptors, which differs between the three patterns of DME. The corresponding anatomical changes in the number and morphology of photoreceptors should be investigated with further histologic or imaging studies.
Code availability
N/A.
References
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY, Meta-Analysis for Eye Disease (META-EYE) Study Group (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564
Klein R, Klein BE, Moss SE (1989) The Wisconsin epidemiological study of diabetic retinopathy: a review. Diabetes Metab Rev 5:559–570
Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R, Verges R (2016) Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res 2016:2156273
Whitmire W, Al-Gayyar MM, Abdelsaid M, Yousufzai BK, El-Remessy AB (2011) Alteration of growth factors and neuronal death in diabetic retinopathy: what we have learned so far. Mol Vis 17:300–308
Guyon B, Elphege E, Flores M, Gauthier AS, Delbosc B, Saleh M (2017) Retinal reflectivity measurement for cone impairment estimation and visual assessment after diabetic macular edema resolution (RECOVER-DME). Invest Ophthalmol Vis Sci 58:6241–6247
Otani T, Kishi S, Maruyama Y (1999) Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol 127:688–693
Staurenghi G, Sadda S, Chakravarthy U, Spaide RF, International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel (2014) Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN•OCT consensus. Ophthalmology 121:1572–1578
Lally DR, Shah CP, Heier JS (2016) Vascular endothelial growth factor and diabetic macular edema. Surv Ophthalmol 61:759–768
Mitchell P, Wong TY, Diabetic Macular Edema Treatment Guideline Working Group (2014) Management paradigms for diabetic macular edema. Am J Ophthalmol 157:505–513
Kodjikian L, Bellocq D, Mathis T (2018) Pharmacological management of diabetic macular edema in real-life observational studies. Biomed Res Int 2018:8289253
Kim M, Lee P, Kim Y, Yu SY, Kwak HW (2011) Effect of intravitreal bevacizumab based on optical coherence tomography patterns of diabetic macular edema. Ophthalmologica 226:138–144
Koytak A, Altinisik M, Sogutlu Sari E, Artunay O, Umurhan Akkan JC, Tuncer K (2013) Effect of a single intravitreal bevacizumab injection on different optical coherence tomographic patterns of diabetic macular oedema. Eye 27:716–721
Seo KH, Yu SY, Kim M, Kwak HW (2016) Visual and morphologic outcomes of intravitreal ranibizumab for diabetic macular edema based on optical coherence tomography patterns. Retina 36:588–595
Ercalik NY, Imamoglu S, Turkseven Kumral E, Yenerel NM, Bardak H, Bardak Y (2018) Influence of serous retinal detachment on the outcome of ranibizumab treatment in diabetic macular oedema. Cutan Ocul Toxicol 37:324–327
Mori Y, Suzuma K, Uji A, Ishihara K, Yoshitake S, Fujimoto M, Dodo Y, Yoshitake T, Miwa Y, Murakami T (2016) Restoration of foveal photoreceptors after intravitreal ranibizumab injections for diabetic macular edema. Sci Rep 6:39161
Spaide RF, Curcio CA (2011) Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina 31:1609–1619
Murakami T, Nishijima K, Akagi T, Uji A, Horii T, Ueda-Arakawa N, Muraoka Y, Yoshimura N (2012) Optical coherence tomographic reflectivity of photoreceptors beneath cystoid spaces in diabetic macular edema. Invest Ophthalmol Vis Sci 53:1506–1511
Serizawa S, Ohkoshi K, Minowa Y, Soejima K (2016) Interdigitation zone band restoration after treatment of diabetic macular edema. Curr Eye Res 41:1229–1234
Bunt-Milam AH, Saari JC, Klock IB, Garwin GG (1985) Zonulae adherentes pore size in the external limiting membrane of the rabbit retina. Invest Ophthalmol Vis Sci 26:1377–1380
Ota M, Nishijima K, Sakamoto A, Murakami T, Takayama K, Horii T, Yoshimura N (2010) Optical coherence tomographic evaluation of foveal hard exudates in patients with diabetic maculopathy accompanying macular detachment. Ophthalmology 117:1996–2002
Theodossiadis PG, Grigoropoulos VG, Theodossiadis GP (2011) The significance of the external limiting membrane in the recovery of photoreceptor layer after successful macular hole closure: a study by spectral domain optical coherence tomography. Ophthalmologica 225:176–184
Grigoropoulos VG, Theodossiadis GP, Theodossiadis PG (2011) Association of the preoperative photoreceptor layer defect as assessed by optical coherence tomography with the functional outcome after macular hole closure: a long follow-up study. Ophthalmologica 225:47–54
Theodossiadis GP, Grigoropoulos VG, Liarakos VS, Rouvas A, Emfietzoglou I, Theodossiadis PG (2012) Restoration of the photoreceptor layer and improvement of visual acuity in successfully treated optic disc pit maculopathy: a long follow-up study by optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 250:971–979
Ito S, Miyamoto N, Ishida K, Kurimoto Y (2013) Association between external limiting membrane status and visual acuity in diabetic macular oedema. Br J Ophthalmol 97:228–232
Ramon y Cajal S (1928) Degeneration and regeneration of the nervous system. Oxford University Press, Oxford
Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D (2000) Retinal stem cells in the adult mammalian eye. Science 287:2032–2036
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
Irini Chatziralli designed the study, collected data, performed the statistical analysis, interpreted data and drafted the manuscript. George Theodossiadis conceived the study, designed the study, interpreted data and drafted the manuscript. Eleni Dimitriou and Dimitrios Kazantzis collected data. Panagiotis Theodossiadis collected data and supervised the study. All authors have read, critically revised and approved the current version of the manuscript.
Corresponding author
Ethics declarations
Availability of data and material
All data will be available upon request.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria, educational grants, or other equity interest), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Consent to participate
All participants gave written informed consent for participation in the study.
Consent for publication
All participants gave written informed consent for their data to be published.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of Attikon Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chatziralli, I., Theodossiadis, G., Dimitriou, E. et al. Association between the patterns of diabetic macular edema and photoreceptors’ response after intravitreal ranibizumab treatment: a spectral-domain optical coherence tomography study. Int Ophthalmol 40, 2441–2448 (2020). https://doi.org/10.1007/s10792-020-01423-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01423-3