Abstract
Aim
Sodium-glucose co-transporter 2 inhibitors (SGLT-2I) are oral anti-diabetic drugs. We aimed to raise awareness by presenting a difficult and long-lasting DKA case that developed after the addition of dapagliflozin to the treatment.
Case presentation
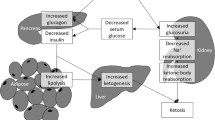
We report a case of a 40-year-old woman who developed diabetic ketoacidosis (DKA) after 2 weeks use of dapagliflozin, a sodium-glucose co-transporter 2 inhibitor (SGLT-2I). She had complaints of nausea, vomiting, loss of consciousness, fever, and shortness of breath. DKA was detected in her laboratory results despite the absence of marked hyperglycemia. The patient had metabolic acidosis episodes accompanied by ketonuria on the 5th (mild) and 9th (severe) day despite discontinuation of dapagliflozin. Sudden fluctuations in blood glucose levels of the patient lasted for 10 days and made it difficult to switch to routine basal-bolus insulin treatment.
Conclusion
Prolonged DKA may be a result of SGLT-2 inhibition and individualized treatment and follow-up should be performed instead of standard DKA treatment. Also, we suggest that we need to evaluate endogenous insulin reserves of the patients before starting a SGLT-2I treatment. We believe that in order to raise awareness, these cases should be reported.

Similar content being viewed by others
References
Fioretto P, Zambon A, Rossato M, et al. SGLT2 inhibitors and the diabetic kidney. Diabetes Care. 2016;39(2):165–71.
Hemmingsen B, Krogh J, Metzendorf MI, et al. Sodium glucose cotransporter (SGLT) 2 inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at risk for the development of type 2 diabetes mellitus. Cochrane Database Syst Rev. 2016;4:CD012106.
Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59.
US Food and Drug Administration. FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Published May 15, 2015. Accessed 2 May 2017.
Goldenberg RM, Berard LD, Cheng AY, et al. SGLT2 inhibitor-associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clin Ther. 2016;38:2654–64.
Monami M, Nreu B, Zannoni S, Lualdi C, Mannucci E. Effects of SGLT-2 inhibitors on diabetic ketoacidosis: a meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2017;130:53–60.
Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638–42.
Sloan G, Kakoudaki T, Ranjan N. Prolonged diabetic ketoacidosis associated with canagliflozin. Endocrinol Diabetes Metab Case Rep. 2018;1:1–5.
Pujara S, Ioachimescu A. Prolonged ketosis in a patient with euglycemic diabetic ketoacidosis secondary to dapagliflozin. JIMHICR. 2017;5:1–4.
Hayami T, Kato Y, Kamiya H, Kondo M, Naito E, Sugiura Y, et al. Case of ketoacidosis by a sodium-glucose cotransporter 2 inhibitor in a diabetic patient with a low-carbohydrate diet. J Diabetes Investig. 2015;6:587–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The authors certify that they have obtained all the appropriate consent forms.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kir, S., Tiken, İ. Prolonged diabetic ketoacidosis and glycemic fluctuations associated with dapagliflozin: a case report. Int J Diabetes Dev Ctries 40, 633–635 (2020). https://doi.org/10.1007/s13410-020-00823-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-020-00823-6