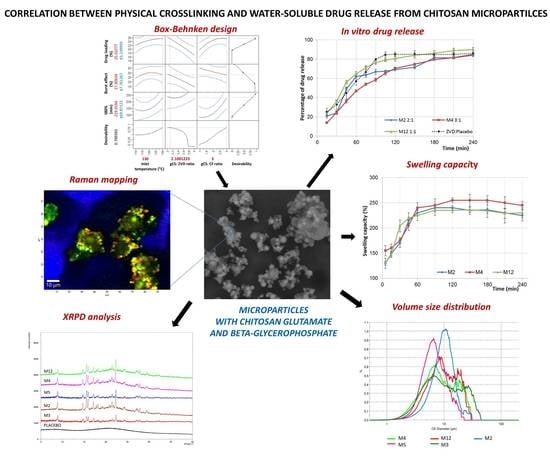
The Correlation between Physical Crosslinking and Water-Soluble Drug Release from Chitosan-Based Microparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spray-Drying Technique
2.3. Microparticles Optimization
2.4. Dissolution Studies
2.5. Microparticles Characterization
2.5.1. Drug and Moisture Content
2.5.2. SEM Analysis
2.5.3. Particle Size Distribution (PSD)
2.5.4. Differential Scanning Calorimetry (DSC)
2.5.5. Raman Confocal Spectroscopy
2.5.6. X-Ray Powder Diffraction (XRPD)
2.5.7. Swelling Capacity Studies
2.6. HPLC Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Dissolution Studies and Microparticles Optimization
3.2. Morphological and Physicochemical Characterization of gCS/CF Microparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Romić, M.D.; Klarić, M.Š.; Lovrić, J.; Pepić, I.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Melatonin-loaded chitosan/Pluronic® F127 microspheres as in situ forming hydrogel: An innovative antimicrobial wound dressing. Eur. J. Pharm. Biopharm. 2016, 107, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Core/shell gel beads with embedded halloysite nanotubes for controlled drug release. Coatings 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Saporito, F.; Sandri, G.; Rossi, S.; Bonferoni, M.C.; Riva, F.; Malavasi, L.; Caramella, C.; Ferrari, F. Freeze dried chitosan acetate dressings with glycosaminoglycans and traxenamic acid. Carbohydr. Polym. 2018, 184, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of chitosan in bone and dental engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nnomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yu, D.; Eom, S.H.; Kim, S.H.; Oh, J.; Jung, W.K.; Kim, Y.M. Synergistic antibacterial effects of chitosan-caffeic acid conjugate against antibiotic-resistant acne-related bacteria. Mar. Drugs 2017, 15, 167. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Cafaggi, S.; Zibana, C.; Donalisio, M.; Cagno, V.; Lembo, D.; Caviglioli, G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 118, 117–125. [Google Scholar] [CrossRef]
- Artan, M.; Karadeniz, F.; Karagozlu, M.Z.; Kim, M.M.; Kim, S.K. Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydr. Res. 2010, 345, 656–662. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E.A. Influence of unmodified and beta-glycerophosphate crosslinked chitosan on anti-Candida activity of clotrimazole in semi-solid delivery systems. Int. J. Mol. Sci. 2014, 15, 17765–17777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignatello, R.; Mangiafico, A.; Pantò, V.; Puglisi, G.; Furneri, P.M. Solid dispersions of chitosan glutamate for the local delivery of miconazole: characterization and in vitro activity. Open Drug Deliv. J. 2008, 2, 44–51. [Google Scholar] [CrossRef]
- Abd-Allah, H.; Kamel, A.O.; Sammour, O.A. Injectable long acting chitosan/tripolyphosphate microspheres for the intra-articular delivery of lornoxicam: Optimization and in vivo evaluation. Carbohydr. Polym. 2016, 149, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Gonzalez-Alvarez, I.; Hernández, M.J.; Corma, A.; Bermejo, M.; Merino, V.; Gonzalez-Alvarez, M. Ionic hydrogel based on chitosan cross-linked with 6-phosphogluconic trisodium salt as a drug delivery system. Biomacromolecules 2018, 19, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Szekalska, M.; Czarnomysy, R.; Lavrič, Z.; Srčič, S.; Miltyk, W.; Winnicka, K. Novel spray dried glycerol 2-phosphate cross-linked chitosan microparticulate vaginal delivery system-development, characterization and cytotoxicity studies. Mar. Drugs 2016, 14, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymańska, E.; Czarnomysy, R.; Jacyna, J.; Basa, A.; Wilczewska, A.Z.; Markuszewski, M.; Winnicka, K. Could spray-dried microbeads with chitosan glutamate be considered as promising vaginal microbicide carriers? The effect of process variables on the in vitro functional and physicochemical characteristics. Int. J. Pharm. 2019, 568, 118558–118570. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination degree of deacetylation of chitosan: Comparison of methods. Prog. Chem. Appl. Chitin Its Deriv. 2012, 17, 5–20. [Google Scholar]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Gupta, J.; Tao, J.Q.; Garg, S.; Al-Kassas, R. Design and development of an in vitro assay for evaluation of solid vaginal dosage forms. Pharmacol. Pharm. 2011, 2, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Rassu, G.; Soddu, E.; Cossu, M.; Brundu, A.; Cerri, G.; Marchetti, N.; Ferraro, L.; Regan, R.F.; Giunchedi, P.; Gavini, E.; et al. Solid microparticles based on chitosan or methyl-β-cyclodextrin: A first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J. Control. Release 2015, 201, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Dunge, A.; Sharda, N.; Singh, B.; Singh, S. Validated specific HPLC method for determination of zidovudine during stability studies. J. Pharm. Biomed. Anal. 2005, 37, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, L.; Galante, J.; Nunes, R.; Sarmento, B.; das Neves, J. Pharmaceutical vehicles for vaginal and rectal administration of anti-HIV microbicide nanosystems. Pharmaceutics 2019, 11, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinatha, A.; Pandit, J.K.; Singh, S. Ionic cross-linked chitosan beads for extended release of ciprofloxacin: In vitro characterization. Indian J. Pharm. Sci. 2008, 70, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Tomaz, A.F.; Sobral de Carvalho, S.M.; Cardoso Barbosa, R.; Silva, S.M.; Gutierrez, M.A.; de Lima, A.G.; Fook, M.V. Ionically crosslinked chitosan membranes used as drug carriers for cancer therapy application. Materials 2018, 11, 2051. [Google Scholar] [CrossRef] [Green Version]
- CN103864869A: Method for Preparing Spherical Zidovudine Crystals. Available online: https://patents.google.com/patent/CN103864869A/en (accessed on 20 March 2020).











| Formulation | Pattern * | gCS:CF Ratio (w/w) | gCS:ZVD Ratio (w/w) | Tin (°C) |
|---|---|---|---|---|
| M1 | 0+− | 2:1 | 3:1 | 110 |
| M2 | 0−− | 2:1 | 2:1 | 110 |
| M3 | −+0 | 1:1 | 3:1 | 120 |
| M4 | +0+ | 3:1 | 2.5:1 | 130 |
| M5 | 0++ | 2:1 | 3:1 | 130 |
| M6 | ++0 | 3:1 | 3:1 | 120 |
| M7 | 000 | 2:1 | 2.5:1 | 120 |
| M8 | 000 | 2:1 | 2.5:1 | 120 |
| M9 | 0−+ | 2:1 | 2:1 | 130 |
| M10 | −0− | 1:1 | 2.5:1 | 110 |
| M11 | 000 | 2:1 | 2.5:1 | 120 |
| M12 | −−0 | 1:1 | 2:1 | 120 |
| M13 | +0− | 3:1 | 2.5:1 | 110 |
| M14 | +−0 | 3:1 | 2:1 | 120 |
| M15 | −0+ | 1:1 | 2.5:1 | 130 |
| Formulation | gCS:CF Ratio (w/w) | gCS:ZVD Ratio (w/w) | ZVD Loading * (%) | Burst Effect * (%) | t80% (min) |
|---|---|---|---|---|---|
| M1 | 2:1 | 3:1 | 17.5 ± 0.3 | 25.9 ± 2.0 | 210 |
| M2 | 2:1 | 2:1 | 23.1 ± 2.0 | 26.0 ± 2.1 | 180 |
| M3 | 1:1 | 3:1 | 14.3 ± 0.3 | 20.3 ± 1.9 | 210 |
| M4 | 3:1 | 2.5:1 | 22.3 ± 0.6 | 23.4 ± 1.1 | 210 |
| M5 | 2:1 | 3:1 | 18.1 ± 1.1 | 15.1 ± 1.2 | 180 |
| M6 | 3:1 | 3:1 | 16.3 ± 1.2 | 26.4 ± 1.9 | 210 |
| M7 | 2:1 | 2.5:1 | 22.6 ± 1.5 | 25.9 ± 1.3 | 105 |
| M8 | 2:1 | 2.5:1 | 20.5 ± 0.8 | 27.4 ± 1.8 | 90 |
| M9 | 2:1 | 2:1 | 24.3 ± 0.9 | 32.7 ± 1.9 | 105 |
| M10 | 1:1 | 2.5:1 | 17.8 ± 1.3 | 21.4 ± 2.1 | 180 |
| M11 | 2:1 | 2.5:1 | 21.9 ± 0.4 | 31.0 ± 1.7 | 90 |
| M12 | 1:1 | 2:1 | 22.6 ± 0.7 | 33.1 ± 2.2 | 120 |
| M13 | 3:1 | 2.5:1 | 19.6 ± 0.9 | 30.4 ± 2.0 | 120 |
| M14 | 3:1 | 2:1 | 25.3 ± 1.0 | 29.8 ± 1.8 | 150 |
| M15 | 1:1 | 2.5:1 | 16.8 ± 0.7 | 22.0 ± 2.4 | 150 |
| Formulation | gCS:CF Ratio (w/w) | gCS:ZVD Ratio (w/w) | Number Distribution (µm) | Volume Distribution (µm) | ||||
|---|---|---|---|---|---|---|---|---|
| D10 | D50 | D90 | D10 | D50 | D90 | |||
| M2 | 2:1 | 2:1 | 0.5 | 1.0 | 5.6 | 5.2 | 9.7 | 15.4 |
| M3 | 1:1 | 3:1 | 1.3 | 2.5 | 5.2 | 3.6 | 8.9 | 26.4 |
| M4 | 3:1 | 2.5:1 | 1.1 | 2.3 | 5.1 | 3.3 | 7.7 | 20.3 |
| M5 | 2:1 | 3:1 | 0.6 | 2.7 | 5.8 | 3.7 | 6.5 | 11.9 |
| M12 | 1:1 | 2:1 | 0.5 | 1.6 | 5.6 | 4.3 | 9.2 | 19.7 |
| Formulation | gCS:CF Ratio (w/w) | ZVD ΔHMP [J/g] | %DC * |
|---|---|---|---|
| M2 | 2:1 | 52.9 ± 1.2 | 51 |
| M3 | 1:1 | 21.1 ± 0.5 | 21 |
| M4 | 3:1 | 45.9 ± 1.6 | 45 |
| M5 | 2:1 | 54.0 ± 1.0 | 52 |
| M12 | 1:1 | 38.0 ± 0.1 | 37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, E.; Woś-Latosi, K.; Jacyna, J.; Dąbrowska, M.; Potaś, J.; Markuszewski, M.J.; Winnicka, K. The Correlation between Physical Crosslinking and Water-Soluble Drug Release from Chitosan-Based Microparticles. Pharmaceutics 2020, 12, 455. https://doi.org/10.3390/pharmaceutics12050455
Szymańska E, Woś-Latosi K, Jacyna J, Dąbrowska M, Potaś J, Markuszewski MJ, Winnicka K. The Correlation between Physical Crosslinking and Water-Soluble Drug Release from Chitosan-Based Microparticles. Pharmaceutics. 2020; 12(5):455. https://doi.org/10.3390/pharmaceutics12050455
Chicago/Turabian StyleSzymańska, Emilia, Katarzyna Woś-Latosi, Julia Jacyna, Magdalena Dąbrowska, Joanna Potaś, Michał Jan Markuszewski, and Katarzyna Winnicka. 2020. "The Correlation between Physical Crosslinking and Water-Soluble Drug Release from Chitosan-Based Microparticles" Pharmaceutics 12, no. 5: 455. https://doi.org/10.3390/pharmaceutics12050455