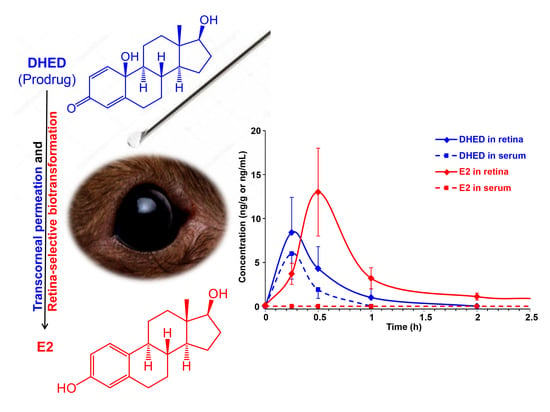
Retina-Targeted Delivery of 17β-Estradiol by the Topically Applied DHED Prodrug
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Corneal Permeability and In Vitro Metabolism Studies
2.4. Topical Drug Treatment and Sample Preparation for Drug Quantitation
2.5. LC-MS Analyses
2.6. Statistical Analyses
3. Results
3.1. Transcorneal Permeability and In Vitro Metabolic Stability Studies
3.2. Topical DHED Produces Large Concentration of E2 in the Retina without Peripheral Exposure to the Hormone
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cascio, C.; Deidda, I.; Russo, D.; Guarneri, P. The estrogenic retina: The potential contribution to healthy aging and age-related neurodegenerative diseases of the retina. Steroids 2015, 103, 31–41. [Google Scholar] [CrossRef]
- Nakazawaa, T.; Takahashia, H.; Shimurac, M. Estrogen has a neuroprotective effect on axotomized RGCs through ERK signal transduction pathway. Brain Res. 2006, 1093, 141–149. [Google Scholar] [CrossRef]
- Prokai-Tatrai, K.; Xin, H.; Nguyen, V.; Szarka, S.; Blazics, B.; Prokai, L.; Koulen, P. 17β-Estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol. Pharm. 2013, 10, 3253–3261. [Google Scholar] [CrossRef] [Green Version]
- Pisano, A.; Preziuso, C.; Iommarini, L.; Perli, E.; Grazioli, P.; Campese, A.F.; Maresca, A.; Montopoli, M. Targeting estrogen receptor β as preventive therapeutic strategy for Leber’s hereditary optic neuropathy. Hum. Mol. Genet. 2015, 24, 6921–6931. [Google Scholar] [CrossRef] [Green Version]
- Feola, A.J.; Fu, J.; Allen, R.; Yang, V.; Campbell, I.C.; Ottensmeyer, A.; Ethier, C.R.; Pardue, M. Menopause exacerbates visual dysfunction in experimental glaucoma. Exp. Eye Res. 2019, 186, 107706. [Google Scholar] [CrossRef]
- Pasquale, L.R.; Aschard, H.; Kang, J.H.; Bailey, J.N.C.; Lindström, S.; Chasman, D.I.; Christen, W.G.; Allingham, R.R.; Ashley-Koch, A.; Lee, R.K.; et al. Age at natural menopause genetic risk score in relation to age at natural menopause and primary open-angle glaucoma in a US-based sample. Menopause 2017, 24, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Dewundara, S.S.; Wiggs, J.L.; Sullivan, D.A.; Pasquale, L.R. Is estrogen a therapeutic target for glaucoma? Semin. Ophthalmol. 2016, 31, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Vajaranant, T.S.; Pasquale, L.R. Estrogen deficiency accelerates aging of the optic nerve. Menopause 2012, 19, 942–947. [Google Scholar] [CrossRef] [Green Version]
- Prokai, L.; Prokai-Tatrai, K.; Simpkins, J.; Agarwal, N. Prodrugs for use as ophthalmic agents. U.S. Patent 7,186,707, 6 March 2007. [Google Scholar]
- Wubben, T.J.; Zacks, D.N.; Besirli, C.G. Retinal neuroprotection: Current strategies and future directions. Curr. Opin. Ophthalmol. 2019, 30, 199–205. [Google Scholar] [CrossRef]
- Prokai-Tatrai, K.; Prokai, L. 17β-Estradiol as a neuroprotective agent. In Sex Hormones in Neurodegenerative Processes and Diseases; Drevensek, G., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 21–39. [Google Scholar]
- Raghava, N.; Das, B.C.; Ray, S.K. Neuroprotective effects of estrogen in CNS injuries: Insights from animal models. Neurosci. Neuroecon. 2017, 6, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Munaut, C.; Lambert, V.; Noël, A.; Frankenne, F.; Deprez, M.; Foidart, J.; Rakic, J. Presence of oestrogen receptor type β in human retina. Br. J. Ophthalmol. 2001, 85, 877–882. [Google Scholar] [CrossRef] [Green Version]
- Prokai, L.; Prokai-Tatrai, K.; Perjesi, P.; Simpkins, J.W. Mechanistic insights into the direct antioxidant effects of estrogens. Drug Dev. Res. 2006, 66, 118–125. [Google Scholar] [CrossRef]
- Prokai, L.; Rivera-Portalatin, N.M.; Prokai-Tatrai, K. Quantitative structure-activity relationships predicting the antioxidant potency of 17β-estradiol-related polycyclic phenols to inhibit lipid peroxidation. Int. J. Mol. Sci. 2013, 11, 1443–1454. [Google Scholar] [CrossRef] [Green Version]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid. Med. Cell Longev. 2019, 1, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Morrison, J.C.; Cepurna, W.O.; Johnson, E.C. Modeling glaucoma in rats by sclerosing aqueous outflow pathways to elevate intraocular pressure. Exp. Eye Res. 2015, 141, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Mead, B.; Tomare, S. Evaluating retinal ganglion cell loss and dysfunction. Exp. Eye Res. 2016, 151, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Prokai, L.; Zaman, K.; Nguyen, V.; Prokai-Tatrai, K. 17β-Estradiol delivered in eye drops: Evidence of impact on protein networks and associated biological processes in the rat retina through quantitative proteomics. Pharmaceutics 2020, 12, 101. [Google Scholar] [CrossRef] [Green Version]
- Speed, V.; Roberts, L.N.; Patel, J.P.; Arya, R. Venous thromboembolism and women’s health. Br. J. Haemetol. 2018, 183, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Sherwin, B.B.; Chertkow, H.; Schipper, H.; Ziad, N. A randomized controlled trial of estrogen treatment in men with mild cognitive impairment. Neurobiol. Aging 2011, 32, 1808–1817. [Google Scholar] [CrossRef]
- Prokai, L.; Nguyen, V.; Szarka, S.; Garg, P.; Sabnis, G.; Bimonte-Nelson, H.A.; McLaughlin, K.J.; Talboom, J.S.; Conrad, C.D.; Shughrue, P.J.; et al. The prodrug DHED selectively delivers 17β-estradiol to the brain for treating estrogen-responsive disorders. Sci. Transl. Med. 2015, 7, 297ra113. [Google Scholar] [CrossRef] [Green Version]
- Prokai-Tatrai, K.; Prokai, L. A novel prodrug approach for central nervous system-selective estrogen therapy. Molecules 2019, 24, 4197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merchenthaler, I.; Lane, M.; Sabnis, G.; Brodie, A.; Nguyen, V.; Prokai, L.; Prokai-Tatrai, K. Treatment with an orally bioavailable prodrug of 17β-estradiol alleviates hot flushes without hormonal effects in the periphery. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tschiffely, A.E.; Schuh, R.A.; Prokai-Tatrai, K.; Prokai, L.; Ottinger, M.A. A comparative evaluation of treatments with 17β-estradiol and its brain-selective prodrug in a double-transgenic mouse model of Alzheimer’s disease. Horm. Behav. 2016, 83, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Tschiffely, A.E.; Schuh, R.A.; Prokai-Tatrai, K.; Ottinger, M.A.; Prokai, L. An exploratory investigation of brain-selective estrogen treatment in males using a mouse model of Alzheimer’s disease. Horm. Behav. 2018, 98, 16–21. [Google Scholar] [CrossRef] [PubMed]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain—from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Eng, S.; Ngo, T.; Dass, C.R. Delivery of therapeutics for deep-seated ocular conditions–status quo. J. Pharm. Pharmacol. 2018, 70, 994–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepta, G.; Edelhauser, H.F. Barriers to glaucoma drug delivery. J. Glaucoma 2008, 17, 147–156. [Google Scholar]
- Prokai-Tatrai, K.; Rivera-Portalatin, N.M.; Rauniyar, N.; Prokai, L. A facile microwave-assisted synthesis of p-quinols by lead (IV) acetate oxidation. Lett. Org. Chem. 2007, 4, 265–267. [Google Scholar] [CrossRef]
- Thiel, M.A.; Morlet, N.; Schulz, D.; Edelhauser, H.F.; Dart, J.K.; Coster, D.J.; Williams, K.A. A simple corneal perfusion chamber for drug penetration and toxicity studies. Br. J. Ophthalmol. 2001, 85, 450–453. [Google Scholar] [CrossRef] [Green Version]
- Szarka, S.; Nguyen, V.; Prokai, L.; Prokai-Tatrai, K. Separation of dansylated 17β-estradiol, 17α-estradiol, and estrone on a single HPLC column for simultaneous quantitation by LC-MS/MS. Anal. Bioanal. Chem. 2013, 405, 3399–3406. [Google Scholar] [CrossRef]
- Prokai-Tatrai, K.; Nguyen, V.; Prokai, L. 10β,17α-dihydroxyestra-1,4-dien-3-one: A bioprecursor prodrug preferentially producing 17α-estradiol in the brain for targeted neurotherapy. ACS Chem. Neurosci. 2018, 9, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Prokai, L.; Zharikov, A.D.; Janák, T.; Prokai-Tatrai, K. Exploratory pharmacokinetics and brain distribution study of a neuropeptide FF antagonist by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2000, 14, 2412–2418. [Google Scholar] [CrossRef]
- Koulen, P.; Xin, H.; Nguyen, V.; Prokai-Tatrai, K.; Prokai, L. Unprecedented protection of the retina in a rat model for glaucoma by topical administration of a novel prodrug for 17β-estradiol. Inv. Ophtal. Vis. Sci. 2009, 50, 4327. [Google Scholar]
- Rivera-Portalatin, N.M.; Vera-Serrano, J.L.; Prokai-Tatrai, K.; Prokai, L. Comparison of estrogen-derived ortho-quinone and para-quinol concerning induction of oxidative stress. J. Steroid Biochem. Mol. Biol. 2007, 105, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szarka, S.; Prokai-Tatrai, K.; Prokai, L. Application of screening experimental designs to assess chromatographic isotope effect upon isotope-coded derivatization for quantitative liquid chromatography–mass spectrometry. Anal. Chem. 2014, 86, 7033–7040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Box, G.E.P.; Hunter, S.J.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005; pp. 317–333. [Google Scholar]
- Moiseev, R.V.; Morrison, P.W.J.; Steel, F.; Khutoryanskiy, V.V. Penetration enhancers in ocular drug delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.E.; Peterson, T.J.; Tong, M.H.; Lee, E.J.; Pfaff, L.E.; Hewitt, S.C.; Korach, K.S.; Weiss, J.; Jameson, L.J. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J. Biol. Chem. 2006, 281, 26683–26692. [Google Scholar] [CrossRef] [Green Version]
- Spady, T.J.; McComb, R.D.; Shull, J.D. Estrogen action in the regulation of cell proliferation, cell survival, and tumorigenesis in the rat anterior pituitary gland. Endocrine 1999, 11, 217–233. [Google Scholar] [CrossRef]
- Zernii, E.Y.; Baksheeva, V.E.; Iomdina, E.N.; Averina, O.A.; Permyakov, S.E.; Philippov, P.P.; Zamyatnin, A.A.; Senin, I.I. Rabbit models of ocular diseases: New relevance for classical approaches. CNS Neurol. Disord. Drug Targets 2016, 15, 267–291. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Hollo, G.; Whitson, J.T.; Faulkner, R.; McCue, B.; Curtis, M.; Wieland, H.; Chastain, J.; Sanders, M.; DeSantis, L.; Przydryga, J.; et al. Concentrations of betaxolol in ocular tissues of patients with glaucoma and normal monkeys after 1 month of topical ocular administration. Investig. Ophthal. Visual Sci. 2006, 47, 235–240. [Google Scholar] [CrossRef] [PubMed]





| Species | Vehicle A | Vehicle B | ||
|---|---|---|---|---|
| d3-E2 | DHED | d3-E2 | DHED | |
| Rat | 6.8 ± 1.5 | 69.0 ± 3.3 | 76.5 ± 5.4 | 234.0 ± 11 |
| Rabbit | 17.9 ± 1.4 | 184.3 ± 18.1 | 89.0 ± 5.5 | 383.0 ± 5.7 |
| Pig | 16.7 ± 1.9 | 171.0 ± 28.0 | 105.5 ± 4.3 | 511.2 ± 64.5 |
| Treatment | Serum E2 (pg/mL) |
|---|---|
| Vehicle eye drops (control) | 8 ± 3 |
| DHED eye drops, single dose | 9 ± 4 |
| DHED eye drops 3 weeks, q.d. | 8 ± 5 |
| E2 eye drops 3 weeks, q.d. | 453 ± 98 *,** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokai-Tatrai, K.; Nguyen, V.; De La Cruz, D.L.; Guerra, R.; Zaman, K.; Rahlouni, F.; Prokai, L. Retina-Targeted Delivery of 17β-Estradiol by the Topically Applied DHED Prodrug. Pharmaceutics 2020, 12, 456. https://doi.org/10.3390/pharmaceutics12050456
Prokai-Tatrai K, Nguyen V, De La Cruz DL, Guerra R, Zaman K, Rahlouni F, Prokai L. Retina-Targeted Delivery of 17β-Estradiol by the Topically Applied DHED Prodrug. Pharmaceutics. 2020; 12(5):456. https://doi.org/10.3390/pharmaceutics12050456
Chicago/Turabian StyleProkai-Tatrai, Katalin, Vien Nguyen, Daniel L. De La Cruz, Rebecca Guerra, Khadiza Zaman, Fatima Rahlouni, and Laszlo Prokai. 2020. "Retina-Targeted Delivery of 17β-Estradiol by the Topically Applied DHED Prodrug" Pharmaceutics 12, no. 5: 456. https://doi.org/10.3390/pharmaceutics12050456