Abstract
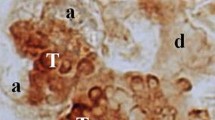
The submandibular gland (SMG) of newborn mice has no mature acini but has the rudiments of acini called terminal tubules (TT). The TT are composed of TT cells with dark secretory granules and proacinar cells with lighter secretory granules, the latter being considered the immediate precursor of mature acinar cells. TT cells contain a specific secretory protein, submandibular gland protein C (SMGC) and they decrease in number postnatally at a higher rate in males than in females. In the present study, in order to clarify the biological roles of TT cells and their secretory product SMGC, we generated a knockout (KO) mouse strain deficient in SMGC. The KO mice of both sexes grew normally, had normal reproductive capacity and had normal acinar and duct systems in the SMG in adult ages. However, through the neonatal and early postnatal stages, the KO mice were deficient not only in the production of SMGC but also in TT cells. With electron microscopy of the SMG of newborn KO mice, TT cells with characteristic granules were absent and replaced by undifferentiated ductal cells, whereas proacinar cells were normal. These results suggested that the absence of SMGC inhibits the development of TT cells and that the absence of SMGC and TT cells has no notable influence on the postnatal development of the acinar and duct systems in the SMG.





Similar content being viewed by others
References
Aloe L, Alleva E, Bohm A, Levi-Montalcini R (1986) Aggressive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. Proc Natl Acad Sci U S A 83:6184–6187
Ball WD, Redman RS (1984) Two independently regulated secretory systems within the acini of the submandibular gland of the perinatal rat. Eur J Cell Biol 33:112–122
Ball WD, Hand AR, Moreira JE, Johnson AO (1988) A secretory protein restricted to type I cells in neonatal rat submandibular glands. Dev Biol 129:464–475
Caramia F (1966) Ultrastructure of mouse submaxillary gland. I Sexual differences. J Ultrastruct Res 16:505–523
Chai Y, Klauser DK, Denny PA, Denny PC (1993) Proliferative and structural differences between male and female mouse submandibular glands. Anat Rec 235:303–311
Chang WW (1974) Cell population changes during acinus formation in the postnatal rat submandibular gland. Anat Rec 178:187–201
Culp DJ, Latchney LR, Fallon MA, Denny PA, Denny PC, Couwenhoven RI, Chuang S (2004) The gene encoding mouse Muc19: cDNA, genomic organization and relationship to Smgc. Physiol Genomics 19:303–318
Das B, Cash MN, Hand AR, Shivazad A, Grieshaber SS, Robinson B, Culp DJ (2010) Tissue distribution of murine Muc19/Smgc gene products. J Histochem Cytochem 58:141–156
Denny PA, Pimprapaiporn W, Kim MS, Denny PC (1988) Quantitation and localization of acinar cell-specific mucin in submandibular glands of mice during postnatal development. Cell Tissue Res 251:381–386
Denny PC, Ball WD, Redman RS (1997) Salivary glands: paradigm for diversity of gland development. Crit Rev Oral Biol Med 8:51–75
Dvorak M (1969) The secretory cells of the submandibular gland in the perinatal period of development in the rat. Z Zellforsch 99:346–356
Gresik EW, MacRae EK (1975) The postnatal development of the sexually dimorphic duct system and of amylase activity in the submandibular glands of mice. Cell Tissue Res 157:411–422
Hayashi H, Ozono S, Watanabe K, Nagatsu I, Onozuka M (2000) Morphological aspects of the postnatal development of submandibular glands in male rats: involvement of apoptosis. J Histochem Cytochem 48:695–698
Hecht R, Connelly M, Marchetti L, Ball WD, Hand AR (2000) Cell death during development of intercalated ducts in the rat submandibular gland. Anat Rec 258:349–358
Jacoby F, Leeson CR (1959) The post-natal development of the rat submaxillary gland. J Anat 93:201–220
Kaneko T (2017) Genome editing in mouse and rat by electroporation. Methods Mol Biol 1630:81–89
Kaneko T, Mashimo T (2015) Simple genome editing of rodent intact embryos by electroporation. PLoS One 10:e0142755
Kumchantuek T, Nakata H, Sakulsak N, Yamamoto M, Iseki S (2016) Expression and localization of calpain 3 in the submandibular gland of mice. Arch Oral Biol 70:9–15
Moreira JE, Tabak LA, Bedi GS, Culp DJ, Hand AR (1989) Light and electron microscopic immunolocalization of rat submandibular gland mucin glycoprotein and glutamine/glutamic acid-rich proteins. J Histochem Cytochem 37:515–528
Muench O, Ernst B (1964) Comparative studies on the histochemical demonstration of mucopolysaccharides with the alcian blue-PAS and the periodic acid-diamine reaction. Acta Histochem 18:51–57
Nakata H, Yamamoto M, Kumchantuek T, Adhapanyawanich K, Nishiuchi T, Iseki S (2017) Synthesis, localization and possible function of serine (or cysteine) peptidase inhibitor, clade B, member 6a (Serpinb6a) in mouse submandibular gland. Cell Tissue Res 369:513–526
Penschow JD, Coghlan JP (1993) Secretion of glandular kallikrein and renin from the basolateral pole of mouse submandibular duct cells: an immunocytochemical study. J Histochem Cytochem 41:95–103
Srivastava HC (1976) Development of acinar cells in the rat submandibular gland. J Anat 123(2):459–465
Yamamoto M, Nakata H, Kumchantuek T, Sakulsak N, Iseki S (2016) Immunohistochemical localization of keratin 5 in the submandibular gland in adult and postnatal developing mice. Histochem Cell Biol 145:327–339
Yamamoto M, Nakata H, Kumchantuek T, Adhapanyawanich K, Iseki S (2018) Distinct hormonal regulation of two types of sexual dimorphism in submandibular gland of mice. Cell Tisse Res 371:261–272
Yamashina S, Barka T (1972) Localization of peroxidase activity in the developing submandibular gland of normal and isoproterenol-treated rats. J Histochem Cytochem 20:855–872
Yamashina S, Barka T (1973) Development of endogenous peroxidese in fetal rat submandibular gland. J Histochem Cytochem 21:42–50
Zinzen KM, Hand AR, Yankova M, Ball WD, Mirels L (2004) Molecular cloning and characterization of the neonatal rat and mouse submandibular gland protein SMGC. Gene 334:23–33
Acknowledgments
We thank Shuichi Yamazaki and Yoshitake Shiraishi for their technical assistance in producing paraffin and epon sections, respectively.
Funding
This study was supported by JSPS KAKENHI Grant Number JP 17K08510 to SI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. The present animal research protocols were approved by the Committee for Animal Experiments in Kanazawa University (approval number: AP-183926).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakata, H., Terakawa, J., Horike, Si. et al. The lack of terminal tubule cells in the submandibular gland of mice deficient in submandibular gland protein C. Cell Tissue Res 381, 229–237 (2020). https://doi.org/10.1007/s00441-020-03205-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03205-w