Abstract
Background
The greatest challenge of the day is the rising threat of neonatal sepsis as well as antimicrobial resistance. Accordingly, attention has been especially devoted to marine macroalgae metabolites, which are known to have prominent medicinal properties with major phytoconstituents. The object of this study is to evaluate the efficacy of three different macroalgal extracts (ME) as new ingredients classes exposed against multidrug-resistant (MDR) Klebsiella pneumoniae isolated from blood specimens of neonatal sepsis, as well as to evaluate the bio-compounds present in ME using gas chromatography–mass spectrometry (GC–MS).
Materials and Methods
Out of 370 neonates, only patients with positive blood culture 110 (29.7%) were enrolled. From clinical specimens, Klebsiella pneumoniae (n = 40) were recovered and were biochemically identified. Antimicrobial susceptibilities of the selected isolates to different antimicrobials were conducted using disk diffusion methods. Antibacterial activities of different AE, Ulva fasciata, Sargassum vulgare, Corallina officinalis, were investigated using agar well diffusion method. Furthermore, the constituents of the most promising ME were detected using GC–MS analysis.
Results
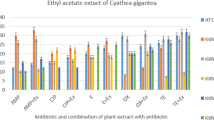
Susceptibility testing showed that all K. pneumoniae isolates were MDR with a great prevalence of resistance, especially to ampicillin and amoxicillin/clavulanic acid. Methanolic extract of S. vulgare exhibited the highest antibacterial activities. GC–MS data of the selected extract revealed eleven different secondary metabolites, which were mostly composed of organic acids.
Conclusion
The methanolic extract of the marine alga S. vulgare could be a potential candidate for the natural compounds as antibacterial therapy.

Similar content being viewed by others
References
Seale, A.C.; Blenocowe, H.; Manu, A.A.; Nair, H.; Bahl, R.; Qazi, S.A.; Zaidi, A.K.; Berkley, J.A.; Cousens, S.N.; Lawn, J.E.: pSBI Investigator Group: estimates of possible severe bacterial infection in neonates in sub-Saharan Africa, South Asia, and Latin American for 2012: a systematic review and meta-analysis. Lancet Infect. Dis 14, 731–741 (2014)
Wynn, J.L.: Defining neonatal sepsis. Curr. Opin. Pediatr. 28, 135–140 (2016)
Hornik, C.P.; Fort, P.; Clark, R.H.; Watt, K.; Benjamin Jr., D.K.; Smith, P.B.; Manzoni, P.; Jacqz-Aigrain, E.; Kaguelidou, F.; Cohen-Wolkowiez, M.: Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum. Dev. (2012). https://doi.org/10.1016/s0378-3782(12)70019-1
Qazi, S.A.; Stoll, B.J.: Neonatal sepsis: a major global public health challenge. Pediatr. Infect. Dis. J. (2009). https://doi.org/10.1097/INF.0b013e31819587a9
Zakariya, B.P.; Vishnu, B.B.; Harish, B.N.; Arun, B.T.; Joseph, N.M.: Risk factors and outcome of Klebsiella pneumoniae sepsis among Newborns. Curr. Pediatr. Res. 16, 115–118 (2012)
Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Davies, H.D.: Early-onset neonatal sepsis. Clin. Microbiol. Rev. (2014). https://doi.org/10.1128/cmr.00031-13
Dong, Y.; Speer, C.P.: Late-onset neonatalsepsis: recent developments. Arch. Dis. Child. Fetal Neonatal Ed. 100, 257–263 (2015)
Sheikh, H.; El-Naggar, A.; Al-Sobahi, D.: Evaluation of Antimycotic Activity of Extracts of Marine Algae Collected from Red Sea Coast, Jeddah. Saudi Arabia. J. Biosci Med. 6, 51–68 (2018)
Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsi, F.; Pataro, G.; Dias, D.A.; Juliano, P.: Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs. (2016). https://doi.org/10.3390/md14110214
Pérez, M.J.; Falqué, E.; Domínguez, H.: Antimicrobial action of compounds from marine seaweed. Mar. Drugs. (2016). https://doi.org/10.3390/md14030052
Singh, R.P.; Kumari, P.; Reddy, C.R.: Antimicrobial compounds from seaweeds-associated bacteria and fungi. Appl. Microbiol. Biotechnol. 99, 1571–1586 (2015)
Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R.: Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. (2017). https://doi.org/10.1016/j.foodres
Nova, N.S.; Uddin, M.D.; Ahmed, T.: Comparative study of the antibacterial activity of seaweed (Sargassum muticm) and freshwater weed (Spirodela polyrrhiza). Bacterial Empire 2, 80–85 (2019)
Aleem, A.A.: The marine Algae of Alexandria, Egypt. In: Aleem AA (Ed.), Faculty of science, University of Alexandria, Egypt (1993)
Jha, B.; Reddy, C.R.K.; Thakur, M.C.; Rao, M.U.: Seaweeds of India. The Diversity and Distribution of Seaweeds of the Gujarat Coast. Springer, London (2009)
Guiry, M.D.; Guiry, G.M.: Algae Base. World-wide electronic publication, National University of Ireland, Galway. (2018) http://www.algaebase.org
Patra, J.K.; Rath, S.K.; Jena, K.B.; Rathod, V.K.; Thatoi, H.: Evaluation of antioxidant and antimicrobial activity of seaweed (Sargassum sp.) extract: a study on inhibition of Glutathione-S transferase activity. Turk. J. Biol. 32, 119–125 (2008)
Roy, S.; Gaind, R.; Chellani, H.; Mohanty, S.; Datta, S.; Singh, A.K.; Basu, S.: Neonatal septicaemia caused by diverse clones of Klebsiella pneumonia and Escherichia coli harbouring blaCTX-M-15. Indian J. Med. Res. 137, 791–799 (2013)
CLSI. Performance Standards for Antimicrobial Susceptibility Tests. 13th ed. CLSI standard M02. Wayne, PA: Clinical and Laboratory Standards Institute (2018)
The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters, version 10.0 (2020)
Holder, I.A.; Boyce, S.T.: Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns 20, 426–429 (1994)
Rahman, M.; Kühn, I.; Rahman, M.; Olsson-Liljequist, B.; Mölby, R.: Evaluation of a scanner- assisted colorimetric MIC method for susceptibility testing of Gram-negative fermentative bacteria. Appl. Environ. Microbiol. 70, 2398–2403 (2004)
Awoniyi, D.O.; Udo, S.J.; Oguntibeju, O.O.: An epidemiological survey of neonatal sepsis in a hospital in Western Nigeria. Afr. J. Microbiol. Res. 3, 385–389 (2009)
Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Davies, H.D.: Early-onset neonatal sepsis. Clin. Microbiol. Rev. 27, 21–47 (2014)
Lamba, M.; Sharma, R.; Sharma, D.; Choudhary, M.; Maheshwari, R.K.: Bacteriological spectrum and antimicrobial susceptibility pattern of neonatal septicaemia in a tertiary care hospital of North India. J. Matern. Fetal Neonatal Med. 29, 3993–3998 (2016)
Muley, V.A.; Ghadage, D.P.; Bhore, A.V.: Bacteriological profile of neonatal septicemia in a tertiary care hospital from western India. J. Global Infect. Dis. 7, 75–77 (2015)
Thakur, S.; Thakur, K.; Sood, A.; Chaudhary, S.: Bacteriological profile and antibiotic sensitivity pattern of neonatal septicaemia in a rural tertiary care hospital in North India. Indian J. Med. Microbiol. 34, 67–71 (2016)
Aletayeb, S.M.H.; Khosravi, A.D.; Dehdashtian, M.; Kompani, F.; Mortazavi, S.M.; Aramesh, M.R.: Identification of bacterial agents and antimicrobial susceptibility of neonatal sepsis: a 54-month study in a tertiary hospital. Afr. J. Microbiol. Res. 5, 528–531 (2011)
Kumar, G.D.; Ramachandran, V.G.; Gupta, P.: Bacteiological analysis of blood culture isolates from neonates in a Tertiary Care Hospital in India. J. Health Popul. Nutr. 20, 343–347 (2002)
World Health Organization: Neonatal and Perinatal Mortality: Country, Regional and Global Estimates. WHO, Geneva (2006)
Lim, N.L.; Wong, Y.H.; Boo, N.Y.; Kasim, M.S.; Chor, C.Y.: Bacteraemic infections in a neonatal intensive care unit: a nine months survey. Med. J. Malaysia 50, 59–63 (1995)
Versporten, A.; Bielicki, J.; Drapier, N.; Sharland, M.; Goossens, H.; ARPEC project group: The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J. Antimicrob. Chemother. 71, 1106–1117 (2016)
Obiero, C.W.; Seale, A.C.; Berkley, J.A.: Empiric treatment of neonatal sepsis in developing countries. Pediatr. Infect. Dis. J. 34, 659–661 (2015)
WHO. World Health Organization, Managing possible serious bacterial infection in young infants when referral is not possible (2015)
Fuchs, A.; Bielicki, J.; Mathur, S.; Sharland, M.R.; Van Den Anker, J.N.: Antibiotic Use for Sepsis in Neonates and Children: 2016 Evidence Update (2017)
Radhika, D.; Veerabahu, C.; Priya, R.: Antibacterial activity of some selected seaweeds from the Gulf of Mannar coast, South India. Asian J. Pharma. Clin. Res. 5, 89–90 (2012)
El Shafay, S.M.; Ali, S.S.; El-Sheekh, M.: Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt. J. Aquat. Res. 42, 65–74 (2016)
Martins, R.M.; Nedel, F.; Guimarães, V.B.S.; da Silva, A.F.; Colepicolo, P.; de Pereira, C.M.P.; Lund, R.G.: Macroalgae extracts from antarctica have antimicrobial and anticancer potential. Front. Microbiol. (2018). https://doi.org/10.3389/fmicb.2018.00412
Manivannan, K.; Karthikai Devi, G.; Anatharaman, P.; Balasubramanian, T.: Antimicrobial potential of selected brown seaweeds from Vedalai coastal waters, Gulf of Mannar. Asian Pac. J. Trop. Biomed. 1, 114–120 (2011)
Yuan, Y.V.: Marine algal constituents. In: Barrow, C.; Shahidi, F. (eds.) Marine nutraceuticals and functional foods, pp. 259–296. CRC, Boca Raton (2008)
Shannon, E.; Abu-Ghannam, N.: Antibacterial derivatives of marine algae: an overview of pharmacological mechanisms and applications. Mar. Drug. (2016). https://doi.org/10.3390/md14040081
Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H.: Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycol. 29, 949–982 (2017)
Cardoso, S.M.; Pereira, O.R.; Seca, A.M.; Pinto, D.C.; Silva, A.M.: Seaweeds as preventive agents for cardiovascular diseases: from nutrients to functional foods. Mar. Drugs 13, 6838–6865 (2015)
Cox, S.; Abu-Ghannam, N.; Gupta, S.: An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 17, 205–220 (2009)
Pradhan, J.; Das, S.; Das, B.K.: Antibacterial activity of freshwater microalgae: A review. Afr. J. Pharm. Pharmacol. 8, 809–818 (2014)
El-Shouny, W.A.; Gaafar, R.M.; Ismai, G.A.; Elzanaty, M.M.: Seasonal variation of the antibacterial activity of some seaweeds against multidrug resistant pathogenic bacterial strains Egypt. J. Exp. Biol. (Bot.) 13, 341–351 (2017)
Acknowledgements
Authors thank Dr. Heba Badier, Pediatrics Department, Faculty of Medicine, Tanta University, Egypt, for helping with the collection of blood specimens from diagnosed neonatal patients.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalil, M., Ismail, M.M. & El Shafay, S.M. Evaluation of Antibacterial Activity of Macroalgae Extracts as Adjunctive Therapy in Neonates Sepsis Induced by Klebsiella pneumoniae. Arab J Sci Eng 45, 4599–4607 (2020). https://doi.org/10.1007/s13369-020-04602-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04602-7