Abstract
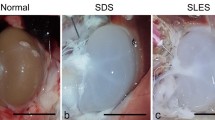
The most preferred decellularization technique in creating bioscaffolds for complex organs such as kidneys is through detergent perfusion. Detergents such as sodium dodecyl sulfate (SDS) flow to the kidneys to remove cells but using this technique alone requires long treatment times. Coupling this technique with sonication treatment decreases decellularization time but may cause damages in the microarchitecture of the kidney. This study evaluated the effects of decellularization parameters specifically SDS concentration (0.25%, 0.625%, and 1.0%wt/vol), flowrate (15, 30, and 45 mL/min), and sonicator power (0, 60, and 120 W) on the length of time needed to produce acellular and intact bioscaffolds. Decellularization was carried out by perfusing SDS to the renal artery of the cadaveric porcine kidney while exposed to sonication treatment. Results showed that a significant decrease in decellularization time was observed in producing acellular scaffold when perfusion decellularization was coupled with sonication. In addition, SDS concentration, SDS flowrate, and sonicator power had significant effects on the decellularization time while only sonicator power had a significant effect on the microarchitecture integrity of the scaffold. Lastly, H&E results showed that the produced bioscaffold showed complete cell removal with only minimal to moderate disruptions on the microarchitecture of the kidney.








Similar content being viewed by others
References
Liyanage, T., Ninomiya, T., & Jha, V. (2015). Worldwide Access to Treatment for End Stage Kidney Disease: A Systematic Review. Journal of Vascular Surgery, 62(4), 1089.
Montserrat, N., Garreta, E., & Belmonte, J. C. I. (2016). Regenerative strategies for kidney engineering. The FEBS Journal, 283(18), 3303-3324.
Lin, Y., Wang, L., Pan, L., Wang, H., Zhu, G., Liu, W., Wang, J. T., Braddock, M., & Zheng, M. (2015). Kidney bioengineering in regenerative medicine: an emerging therapy for kidney disease. Cytotherapy, 18(2), 186–197.
Destefani, A. C., Sirtoli, G. M., & Nogueira, B. V. (2017). Advances in the knowledge about kidney decellularization and repopulation. Frontiers in Bioengineering and Biotechnology, 5, 34.
Ross, E. A., Williams, M. J., Hamazaki, T., Terada, N., Clapp, W. L., Adin, C., Ellison, G. W., Jorgensen, M., & Batich, C. D. (2009). Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. Journal of the American Society of Nephrology, 20(11), 2338–2347.
Crapo, P. M., Gilbert, T. W., & Badylak, S. F. (2011). An overview of tissue and whole organ decellularization processes. Biomaterials, 32(12), 3233–3243.
Peloso, A., Tamburrini, R., Edgar, L., Wilm, B., Katari, R., Perin, L., Murray, P., & Orlando, G. (2016). Extracellular matrix scaffolds as a platform for kidney regeneration. European Journal of Pharmacology, 790, 21–27.
Abolbashari, M., Agcaoili, S.M., Lee, M.K., Ko, I.K., Aboushwareb, T., Jackson, J.D., Yoo, J.J. and Atala, A. (2016). Repopulation of porcine kidney scaffold using porcine primary renal cells. Acta Biomaterialia, 52–61.
Sullivan, D. C., Mirmalek-Sani, S. H., Deegan, D. B., Baptista, P. M., Aboushwareb, T., Atala, A., & Yoo, J. J. (2012). Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials, 33(31), 7756-7764.
Guyette, J. P., Gilpin, S. E., Charest, J. M., Tapias, L. F., Ren, X., & Ott, H. C. (2014). Perfusion decellularization of whole organs. Nature Protocols, 9(6), 1451–1468.
Guan, Y., Liu, S., Liu, Y., Sun, C., Cheng, G., Luan, Y., Li, K., Wang, J., Xie, X. and Zhao, S. (2015). Porcine kidneys as a source of ECM scaffold for kidney regeneration. Materials Science and Engineering: C, 56, 451-456.
Poornejad, N., Momtahan, N., Salehi, A.S., Scott, D.R., Fronk, C.A., Roeder, B.L., Reynolds, P.R., Bundy, B.C. and Cook, A.D. (2016). Efficient decellularization of whole porcine kidneys improves reseeded cell behavior. Biomedical Materials, 11(2), 025003.
Caralt, M., Uzarski, J.S., Iacob, S., Obergfell, K.P., Berg, N., Bijonowski, B.M., Kiefer, K.M., Ward, H.H., Wandinger‐Ness, A., Miller, W.M. and Zhang, Z.J. (2015). Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. American Journal of Transplantation, 15(1), 64-75.
Wainwright, J. M., Czajka, C. A., Patel, U. B., Freytes, D. O., Tobita, K., Gilbert, T. W., & Badylak, S. F. (2010). Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Engineering Part C: Methods, 16(3), 525-532.
Hashimoto, Y., Funamoto, S., Sasaki, S., Honda, T., Hattori, S., Nam, K., Kimura, T., Mochizuki, M., Fujisato, T., Kobayashi, H. and Kishida, A. (2010). Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials, 31(14), 3941-3948.
Funamoto, S., Nam, K., Kimura, T., Murakoshi, A., Hashimoto, Y., Niwaya, K., Kitamura, S., Fujisato, T. and Kishida, A. (2010). The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials, 31(13), 3590-3595.
Hung, S. H., Su, C. H., Lee, F. P., & Tseng, H. (2013). Larynx decellularization: combining freeze-drying and sonication as an effective method. Journal of Voice, 27(3), 289-294.
Azhim, A., Ono, T., Fukui, Y., Morimoto, Y., Furukawa, K., & Ushida, T. (2013, July). Preparation of decellularized meniscal scaffolds using sonication treatment for tissue engineering. In 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 6953-6956). IEEE.
Azhim, A., Syazwani, N., Morimoto, Y., Furukawa, K. S., & Ushida, T. (2014). The use of sonication treatment to decellularize aortic tissues for preparation of bioscaffolds. Journal of Biomaterials Applications, 29(1), 130-141.
Say, S., Dugos, N., Roces, S., & Mondragon, J. M. (2019). Effect of sonication power on perfusion decellularization of cadaveric porcine kidney. In MATEC Web of Conferences (Vol. 268, p. 01009). EDP Sciences.
Fischer, I., Westphal, M., Rossbach, B., Bethke, N., Hariharan, K., Ullah, I., Reinke, P., Kurtz, A. and Stachelscheid, H. (2017). Comparative characterization of decellularized renal scaffolds for tissue engineering. Biomedical Materials, 12(4), 045005.
Saranya, N., Devi, P., Nithiyanantham, S., & Jeyalaxmi, R. (2014). Cells disruption by ultrasonication. BioNanoScience, 4(4), 335–337.
Mohd Yusof N.S., Ashokkumar M. (2015) Ultrasonic Modification of Micelle Structures. In: Ashokkumar M. (eds) Handbook of Ultrasonics and Sonochemistry. Springer, Singapore
Park, S. M., Yang, S., Rye, S. M., & Choi, S. W. (2018). Effect of pulsatile flow perfusion on decellularization. Biomedical Engineering Online, 17(1), 15.
Lichtenberg, D., Ahyayauch, H., & Goñi, F. M. (2013). The mechanism of detergent solubilization of lipid bilayers. Biophysical Journal, 105(2), 289-299.
Vaidyanathan, S., Orr, B. G., & Banaszak Holl, M. M. (2014). Detergent induction of HEK 293A cell membrane permeability measured under quiescent and superfusion conditions using whole cell patch clamp. The Journal of Physical Chemistry B, 118(8), 2112–2123.
Motin, M. A., Mia, M. H., & Islam, A. N. (2015). Thermodynamic properties of sodium dodecyl sulfate aqueous solutions with methanol, ethanol, n-propanol and iso-propanol at different temperatures. Journal of Saudi Chemical Society, 19(2), 172–180.
Bertanha, M., Moroz, A., Jaldin, R.G., Silva, R.A., Rinaldi, J.C., Golim, M.A., Felisbino, S.L., Domingues, M.A., Sobreira, M.L., Reis, P.P. and Deffune, E. (2014). Morphofunctional characterization of decellularized vena cava as tissue engineering scaffolds. Experimental Cell Research, 326(1), 103-111.
Funding
This work was funded by the Commission on Higher Education (CHED) Discovery-Applied Research and Extension for Trans/Inter-disciplinary Opportunities (DARETO) Project Research Grant, the Department of Science and Technology Engineering Research and Development for Technology (DOST-ERDT), and the De La Salle University Research and Coordination Office (URCO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The presenting author of this manuscript in ACB2019 is Tosha Mae Manalastas.
Rights and permissions
About this article
Cite this article
Manalastas, T.M., Dugos, N., Ramos, G. et al. Effect of Decellularization Parameters on the Efficient Production of Kidney Bioscaffolds. Appl Biochem Biotechnol 193, 1239–1251 (2021). https://doi.org/10.1007/s12010-020-03338-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03338-2