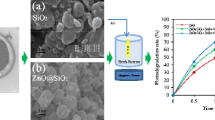
Abstract
A simple microwave method was employed to prepare silica-doped mesoporous zirconia nanoparticles, SiO2/ZrO2 (SiZr) catalysts under various Si amount and then characterized by X-ray diffraction, nitrogen adsorption–desorption analyses, Fourier-transform infrared, electron spin resonance, ultraviolet–visible diffuse reflectance spectroscopy and photoluminescence analyses. The lower amount of Si fully stabilized the ZrO2 in the tetragonal phase (t-ZrO2), but the higher amount of Si occupied oxygen vacancies (OV) in the SiZr lattice to disrupt the catalysts with introduce low content of monoclinic phase. The catalyst activity towards on photodegradation of 2-chlorophenol (2-CP) was ranked in the following order: 1SiZr (92%) > TiO2 (73%) > 2SiZr (67%) > 3SiZr (64%) > 4SiZr (56%) > ZrO2 (51%). This result demonstrated that 1SiZr gave the highest degradation percentage of 10 mg L−1 2-CP at pH 5 using 0.375 g L−1 catalyst under visible light irradation within 4 h. The highest photoactivity of 1SiZr is due to the larger surface area and crystallite size, which resulted in a good surface contact with light and thus accelerated the photocatalytic activity. Additionally, the highest amount of OV possesed by 1 SiZr effectively suppressed the electron–hole recombination by acting as an electron acceptors, which consequently affected the t-ZrO2 stabilization as well as the catalytic activity. The kinetics of photocatalytic degradation of 2-CP correlated with pseudo-first order model, with the surface reaction as the controlling step. The photogenerated hole was the most active species as confirmed by effect of scavenger study. The 1SiZr maintained the photocatalytic activities after five runs and has a great capability in degrading of various phenols derivatives, indicating its potential use in the phenol-based wastewater treatment.
Graphic Abstract










Similar content being viewed by others
References
Azami MS, Jalil AA, Hitam CNC, Hassan NS, Mamat CR, Adnan RH, Chanlek N (2020) Appl Surf Sci 512:145744
Igbinosa EO, Odjadjare EE, Chigor VN, Igbinosa IH, Emoghene AO, Ekhaise FO, Igiehon NO, Idemudia OG (2013) Sci World J 1:1–11
Jusoh R, Jalil AA, Triwahyono S, Idris A, Haron S, Sapawe N, Jaafar NF, Jusoh NWC (2014) Appl Catal A 469:33–44
Sharotri N, Sud D (2016) Desalin Water Treat 57:8776–8788
Peng S, Tang Z, Jiang W, Wu D, Hong S, Xing B (2017) Sci Total Environ 581:550–558
Duan X, Zhao C, Liu W, Zhao X, Chang L (2017) Electrochim Acta 240:424–436
Jaafar NF, Jalil AA, Triwahyono S, Shamsuddin N (2015) RSC Adv 5:90991–91000
Solís-Casados DA, Martínez-Peña J, Hernández-López S, Escobar-Alarcón L (2020) Top Catal 1–11.
Melchor-Lagar V, Ramos-Ramirez E, Morales-Perez A, Rangel-Vazquez I, Angel GD (2020) J Photoch Photobio A 389:112251
Reddy CV, Reddy IN, Akkinepally B, Harish VVN, Reddy KR, Jaesool S (2019) Ceram Int 45:15298–15306
Hassan NS, Jalil AA, Triwahyono S, Hitam CNC, Rahman AFA, Khusnun NF, Prasetyoko D (2018) J Taiwan Inst Chem Eng 82:322–330
Basahel SN, Ali TT, Mokhtar M, Narasimharao K (2015) Nanoscale Res Lett 10:73–86
Gorban O, Synyakina S, Volkova G, Gorban S, Konstantiova T, Lyubchik S (2015) J Solid State Chem 232:249–255
Sudrajat H, Babel S, Sakai H, Takizawa S (2016) J Environ Manag 165:224–234
Agorku ES, Kuvarega AT, Mamba BB, Pandey AC, Mishra AK (2015) J Rare Earths 33:498–506
Gurushantha K, Anantharaju KS, Nagabhushana H, Sharma SC, Vidya YS, Shivakumara C, Anilkumar MR (2015) J Mol Catal A Chem 397:36–47
Renuka L, Anantharaju KS, Sharma SC, Nagaswarupa HP, Prashantha SC, Nagabhushana H, Vidya YS (2016) J Alloys Compd 672:609–622
Poungchan G, Ksapabutr B, Panapoy M (2016) Mater Des 89:137–145
Carevic MV, Abazovic ND, Novakovic TB, Pavlovic VB, Comor MI (2016) Appl Catal B 195:112–120
Hassan NS, Jalil AA, Triwahyono S, Khusnun NF, Izan SM, Kidam K, Johari A (2018) J Mol Liq 261:423–430
Vignesh K, Suganthi A, Min B, Kang M (2015) Appl Surface Sci 324:652–661
Fakhri A, Behrouz S, Tyagi I, Agarwal S, Gupta VK (2016) J Mol Liq 216:342–346
Dwivedi R, Maurya A, Verma A, Prasad R, Bartwal KS (2011) J Alloys Compd 509:6848–6851
Adamski A, Jakubus P, Sojka Z (2007) Solid State Phenom 128:89–96
Gauna MR, Conconi MS, Gomez S, Suarez G, Aglieeti EF, Rendtorff NM (2015) Ceram-Silik 59:318–325
Li P, Chen I (1994) J Am Ceram Soc 77:1289–1295
Hitam CNC, Jalil AA, Triwahyono S, Ahmad A, Jaafar NF, Salamun N, Ghazali Z (2016) RSC Adv 6:76259–76268
Khusnun NF, Jalil AA, Triwahyono S, Hitam CNC, Hassan NS, Jamian F, Hartanto D (2018) Powder Technol 327:170–178
Karim AH, Jalil AA, Triwahyono S, Kamarudin NHN, Ripin A (2014) J Colloid Interface Sci 421:93–102
Hassan NS, Jalil AA, Khusnun NF, Ali MW, Haron S (2019) J Alloys Compd 789:221–230
Jusoh NWC, Jalil AA, Triwahyono S, Karim AH, Salleh NF, Annuar NHR, Jaafar NF, Firmansyah ML, Mukti RR, Ali MW (2015) Appl Surf Sci 330:10–19
Patel A, Singh S (2016) J Taiwan Inst Chem Eng 64:306–313
Hassan NS, Roslani NJ, Jalil AA, Triwahyono S, Salleh NF, Jaafar NF (2015) Mal J Fund Appl Sci 11:148–151
Maheswari AU, Kumar SS, Sivakumar M (2014) Ceram Int 40:6561–6568
Wang H, Yao X, Sui G, Yin L, Wang L (2015) J Mater Sci Technol 31:164–170
Kouva S, Honkala K, Leffertsac L, Kanervo J (2015) Catal. Sci. Technol 5:3473–3490
Siddiquey IA, Furusawa T, Sato M, Bahadur NM, Uddin MN, Suzuki N (2013) Ceram Int 7:1755–1760
Gionco C, Paganini MC, Giamello E, Burgess R, Valentin CD, Pacchioni G (2013) Chem Mater 25:2243–2253
Zhang J, Gao Y, Jia X, Wang J, Chen Z, Xu Y (2018) Sol Energy Mater Sol Cells 182:113–120
Fauzi AA, Jalil AA, Mohamed M, Triwahyono S, Jusoh NWC, Rahman AFA, Tanaka H (2018) J Environ Manag 227:34–43
Hitam CNC, Jalil AA, Triwahyono S, Rahman AFA, Hassan NS, Khusnun NF, Ahmad A (2018) Fuel 216:407–417
Rahman AFA, Jalil AA, Triwahyono S, Ripin A, Aziz FFA, Fatah NAA, Hassan NS (2017) J Clean Prod 143:948–959
Nandiyanto ABD, Zaen R, Oktiani R (2017) Arab J Chem 13:1283–1296
Jaafar NF, Jalil AA, Triwahyono S (2017) Appl Surf Sci 392:1068–1077
Elsalamony RA, Mahmoud SA (2017) Arab J Chem 10:194–205
Khusnun NF, Jalil AA, Triwahyono S, Jusoh NWC, Johari A, Kidam K (2016) Phys Chem Chem Phys 18:12323–12331
Singh AK, Nakate UT (2014) Sci World J 1–7.
Zhou X, Lai C, Huang D, Zeng G, Chen L, Qin L, Xu P, Cheng M, Huang C, Zhang C, Zhou C (2018) J Hazard Mater 346:113–123
Aziz FFA, Jalil AA, Triwahyono S, Mohamed M (2018) Appl Surf Sci 455:84–95
Acknowledgements
The authors are grateful for the financial support by the Universiti Teknologi Malaysia through Professional Development Research University Grant (No. 04E73), Fundamental Research Grant Scheme from Ministry of Higher Education Malaysia (Grant No. FRGS/1/2019/STG07/UTM/01/1 -5F192) and Research University Grant from Universiti Teknologi Malaysia (Grant No. 08G92).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there are no known conflicts of interest associated with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hassan, N.S., Jalil, A.A., Aziz, F.F.A. et al. Tailoring the Silica Amount in Stabilizing the Tetragonal Phase of Zirconia for Enhanced Photodegradation of 2-Chlorophenol. Top Catal 63, 1145–1156 (2020). https://doi.org/10.1007/s11244-020-01274-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-020-01274-3