Abstract
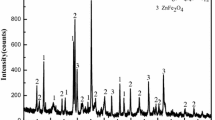
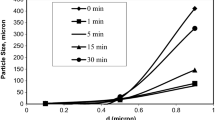
Zinc leaching residue (ZLR) contains high content of valuable metals such as zinc and iron. However, zinc and iron mainly exist in the form of zinc ferrite, which are difficult to separate and recover. This study proposed a new process involving sulfidation roasting, magnetic separation and flotation to recover zinc and iron in ZLR. Through sulfidation roasting of ZLR with pyrite, zinc and iron were converted into ZnS and Fe3O4. The effects of pyrite dosage, roasting temperature and roasting time on the sulfidation of zinc in ZLR were investigated. The results showed that the sulfidation percentage of zinc reached 91.8% under the optimum condition. Besides, it was found that ball-milling was favorable for the separation and recovery of zinc and iron in sulfidation products. After ball-milling pretreatment, iron and zinc were enriched from sulfidation products by magnetic separation and flotation. The grade of iron in magnetic concentrates was 52.3% and the grade of zinc in flotation concentrates was 31.7%, which realized the recovery of resources.
摘要
锌浸渣中含有大量锌, 铁等有价金属,但其中锌和铁主要以铁酸锌等复杂物相形式赋存,. 难以 分离和回收.本研究提出了涉及硫化焙烧、磁选和浮选方法回收锌浸渣中锌和铁的新工艺.以黄铁矿 为硫化剂对锌浸渣进行硫化焙烧,将锌和铁分别转化为硫化锌和四氧化三铁.研究了焙烧温度、焙烧 时间及黄铁矿添加量对锌浸渣中锌的硫化转化的影响.结果表明,在最佳工艺条件下,锌浸渣中锌的 硫化率可达91.8%.此外,研究发现球磨有利于硫化产物中锌、铁的分离和回收.球磨预处理后,通 过磁选和浮选实现硫化产物中铁和锌的富集.铁精矿铁品位达52.3%,锌精矿锌品位达31.7%.可见, 该方法有效地实现了锌浸渣中锌和铁的分离与回收
Similar content being viewed by others
References
ÖZVERDI A, ERDEM M. Environmental risk assessment and stabilization/solidification of zinc extraction residue: I Environmental risk assessment [J]. Hydrometallurgy, 2010, 100(3, 4): 103–109. DOI: https://doi.org/10.1016/j.hydromet.2009.10.011.
EJTEMAEI M, GHARABAGHI M, IRANNAJAD M. A review of zinc oxide mineral beneficiation using flotation method [J]. Adv Colloid Interface Sci, 2014, 1: 68–78. DOI: https://doi.org/10.1016/j.cis.2013.02.003.
KE Yong, CHAI Li-yuan, MIN Xiao-bo, TANG Chong-jian, CHEN Jie, WANG Yan, LIANG Yan-jie. Sulfidation of heavy-metal-containing neutralization sludge using zinc leaching residue as the sulfur source for metal recovery and stabilization [J]. Minerals Engineering, 2014, 1: 105–112. DOI: https://doi.org/10.1016/j.mineng.2014.03.022.
CHEN T T, DUTRIZAC J E. Mineralogical changes occurring during the fluid-bed roasting of zinc sulfide concentrates [J]. The Journal of The Minerals, Metals & Materials Society, 2004, 56(12): 46–51. DOI: https://doi.org/10.1007/s11837-004-0235-y.
YU Gang, PENG Ning, ZHOU Lan, LIANG Yan-jie, ZHOU Xiao-yuan, PENG Bing, CHAI Li-yuan, YANG Zhi-hui. Selective reduction process of zinc ferrite and its application in treatment of zinc leaching residues [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(8): 2744–2752. DOI: https://doi.org/10.1016/S1003-6326(15)63899-7.
LECLERC N, MEUX E, LECUIRE J M. Hydrometallurgical extraction of zinc from zinc ferrites [J]. Hydrometallurgy, 2003, 70(1–3): 175–183. DOI: https://doi.org/10.1016/s0304-386x(03)00079-3.
TURAN M D, SALTUNDOGAN H S, TÜMEN F. Recovery of zinc and lead from zinc plant residue [J]. Hydrometallurgy, 2004, 75(1–4): 169–176. DOI: https://doi.org/10.1016/j.hydromet.2004.07.008.
HOLLOWAY P C, ETSELL T H, MURLAND A L. Roasting of La Oroya zinc ferrite with Na2CO3 [J]. Metallurgical and Materials Transactions B, 2007, 38(5): 781–791. DOI: https://doi.org/10.1007/s11663-007-9082-x.
ZHANG Ya-li, YU Xian-jin, LI Xiao-bin. Zinc recovery from franklinite by sulphation roasting [J]. Hydrometallurgy, 2011, 109(3, 4): 211–214. DOI: https://doi.org/10.1016/j.hydromet.2011.07.002.
LI Chao, SUN Heng-hu, BAI Jing, LI Long-tu. Innovative methodology for comprehensive utilization of iron ore tailings: Part 2: The residues after iron recovery from iron ore tailings to prepare cementitious material [J]. Journal of Hazardous Materials, 2010, 174(1–3): 78–83. DOI: https://doi.org/10.1016/j.jhazmat.2009.09.019.
YU Jian-wen, HAN Yue-xin, LI Yan-jun, GAO Peng. Recent advances in magnetization roasting of refractory iron ores: A technological review in the past decade [J]. Mineral Processing and Extractive Metallurgy Review, 2019. DOI: https://doi.org/10.1080/08827508.2019.1634565.
DENG Jin-chuan, FENG Xin, QIU Xin-hong. Extraction of heavy metal from sewage sludge using ultrasound-assisted nitric acid [J]. Chemical Engineering Journal, 2009, 152(1): 177–182. DOI: https://doi.org/10.1016/j.cej.2009.04.031.
SADEGH S M, BAFGHI M S, MORADKHANI D, ILKHCHI M O. A review on hydrometallurgical extraction and recovery of cadmium from various resources [J]. Minerals Engineering, 2007, 20(3): 211–220. DOI: https://doi.org/10.1016/j.mineng.2006.07.001.
LIANG Yan-jie, CHAI Li-yuan, MIN Xiao-bo, TANG Chong-jian, ZHANG Hai-jing, KE Yong, XIE Xian-de. Hydrothermal sulfidation and floatation treatment of heavy-metal-containing sludge for recovery and stabilization [J]. Journal of Hazardous Materials, 2012, 217–1: 307–314. DOI: https://doi.org/10.1016/j.jhazmat.2012.03.025.
WANG Jun, LU Jin-feng, ZHANG Qi-wu, SAITO F. Mechanochemical sulfidization of nonferrous metal oxides by grinding with sulfur and Iron [J]. Industrial & Engineering Chemistry Research, 2003, 42(23): 5813–5818. DOI: https://doi.org/10.1021/ie030046b.
KUCHAR D, FUKUTA T, ONYANGO M S, MATSUDA H. Sulfidation of zinc plating sludge with Na2S for zinc resource recovery [J]. Journal of Hazardous Materials, 2006, 137(1): 185–191. DOI: https://doi.org/10.1016/j.jhazmat.2006.05.037.
LI Yong, WANG Ji-kun, WEI Chang, LIU Chun-xia, JIANG Ji-bo, WANG Fan. Sulfidation roasting of low grade lead-zinc oxide ore with elemental sulfur [J]. Minerals Engineering, 2010, 23(7): 563–566. DOI: https://doi.org/10.1016/j.mineng.2010.01.004.
KUCHAR D, FUKUTA T, ONYANGO M S, MATSUDA H. Sulfidation treatment of copper-containing plating sludge towards copper resource recovery [J]. Journal of Hazardous Materials, 2006, 138(1): 86–94. DOI: https://doi.org/10.1016/j.jhazmat.2006.01.052.
ZHENG Yong-xin, LIU Wei, QIN Wen-qin, HAN Jun-wei, YANG Kang, LUO Hong-lin, WANG Da-wei. Improvement for sulphidation roasting and its application to treat lead smelter slag and zinc recovery [J]. Canadian Metallurgical Quarterly, 2015, 54(1): 92–100. DOI: https://doi.org/10.1179/1879139514y.0000000155.
MIN Xiao-bo, XUE Ke, KE Yong, ZHOU Bo-sheng, LI-YANG Wen-jun, WANG Qing-wei. Sulfidation roasting of hemimorphite with pyrite for the enrichment of Zn and Pb [J]. The Journal of The Minerals, Metals & Materials Society, 2016, 68(9): 2435–2442. DOI: https://doi.org/10.1007/s11837-016-1986-y.
KE Yong, PENG Ning, XUE Ke, MIN Xiao-bo, CHAI Li-yuan, PAN Qing-lin, LIANG Yan-jie, XIAO Rui-yang, WANG Yun-yan, TANG Chong-jian, LIU Hui. Sulfidation behavior and mechanism of zinc silicate roasted with pyrite [J]. Applied Surface Science, 2018, 1: 1011–1019. DOI: https://doi.org/10.1016/j.apsusc.2017.11.202.
MIN Xiao-bo, ZHOU Bo-sheng, KE Yong, CHAI Li-yuan, XUE Ke, ZHANG Chun, ZHAO Zong-wen, SHEN Chen. Sulfidation behavior of ZnFe2O4 roasted with pyrite: Sulfur inducing and sulfur-oxygen interface exchange mechanism [J]. Applied Surface Science, 2016, 1: 67–73. DOI: https://doi.org/10.1016/j.apsusc.2016.02.229.
MIN Xiao-bo, LI-YANG Wen-jun, KE Yong, SHI Mei-qing, CHAI Li-yuan, XUE Ke. Fe-FeS2 adsorbent prepared with iron powder and pyrite by facile ball milling and its application for arsenic removal [J]. Water Science & Technology, 2017, 76(1): 192–200. DOI: https://doi.org/10.2166/wst.2017.204.
ZHANG Hui-bin. Chemical phase analysis of ore and industrial product [M]. Beijing: Metallurgical Industry Press, 1992. (in Chinese)
KE Yong, CHAI Li-yuan, LIANG Yan-jie, MIN Xiao-bo, YANG Zhi-hui, CHEN Jie, YUAN Sheng. Sulfidation of heavy-metal-containing metallurgical residue in wet-milling processing [J]. Minerals Engineering, 2014, 1: 136–143. DOI: https://doi.org/10.1016/j.mineng.2013.07.013.
FENG D, ALDRICH C. Effect of particle size on flotation performance of complex sulphide ores [J]. Minerals Engineering, 1999, 7(12): 721–731. DOI: https://doi.org/10.1016/s0892-6875(99)00059-x.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2018YFC1900305) supported by the National Key R&D Program of China; Project(51825403) supported by the National Science Foundation for Distinguished Young Scholars, China; Projects(51634010, 51474247, 51904354) supported by the National Natural Science Foundation of China; Project(2019SK2291) supported by the Key Research and Development Program of Hunan Province, China
Rights and permissions
About this article
Cite this article
Min, Xb., Jiang, Gh., Wang, Yy. et al. Sulfidation roasting of zinc leaching residue with pyrite for recovery of zinc and iron. J. Cent. South Univ. 27, 1186–1196 (2020). https://doi.org/10.1007/s11771-020-4359-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-020-4359-1