Abstract
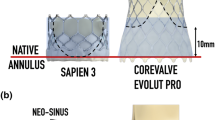
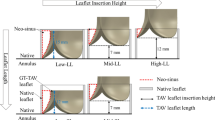
Thrombosis in post-transcatheter aortic valve replacement (TAVR) patients has been correlated with flow stasis in the neo-sinus. This study investigated the effect of the post-TAVR geometry on flow stasis. Computed tomography angiography of 155 patients who underwent TAVR using a SAPIEN 3 were used to identify patients with and without thrombosis, and quantify thrombus volumes. Six patients with 23-mm SAPIEN 3 valves were then selected from the cohort and used to create patient-specific post-TAVR computational fluid dynamic models. Regions of flow stasis (%Volstasis, velocities below 0.05 m/s) were identified. The results showed that all post-TAVR anatomical measurements were significantly different in patients with and without thrombus, but only sinus diameter had a linear correlation with thrombus volume (r = 0.471, p = 0.008). A linear correlation was observed between %Volstasis and thrombus volume (r = 0.821, p = 0.007). The combination of anatomy and valve deployment created a unique geometry in each patient, which when combined with patient-specific cardiac output, resulted in distinct flow patterns. While parametric studies have shown individual anatomical or deployment metrics may relate to flow stasis, the combined effects of these metrics potentially contributes to the biomechanical environment promoting thrombosis, therefore hemodynamic studies of TAVR should account for these patient-specific factors.






Similar content being viewed by others
Abbreviations
- AS:
-
Aortic stenosis
- CAD:
-
Computer aided design
- CFD:
-
Computational fluid dynamics
- CO:
-
Cardiac output
- CTA:
-
Computed tomography angiography
- FSI:
-
Fluid–structure interaction
- HALT:
-
Hypo-attenuated leaflet thickening
- LCA:
-
Left coronary artery
- LVOT:
-
Left ventricular outflow tract
- PIV:
-
Particle image velocimetry
- PLM:
-
Parametric leaflet model
- RCA:
-
Right coronary artery
- SOV:
-
Sinus of Valsalva
- STJ:
-
Sinotubular junction
- TAV:
-
Transcatheter aortic valve
- TAVR:
-
Transcatheter aortic valve replacement
References
Auricchio, F., M. Conti, S. Morganti, and A. Reali. Simulation of transcatheter aortic valve implantation: a patient-specific finite element approach. Comput. Methods Biomech. Biomed. Eng. 17:1347–1357, 2014.
Barth T. and D. Jespersen. The design and application of upwind schemes on unstructured meshes. In: 27th Aerospace Sciences Meeting. American Institute of Aeronautics and Astronautics, 1989.
Bianchi, M., G. Marom, R. P. Ghosh, O. M. Rotman, P. Parikh, L. Gruberg, and D. Bluestein. Patient-specific simulation of transcatheter aortic valve replacement: impact of deployment options on paravalvular leakage. Biomech. Model. Mechanobiol. 18:435–451, 2019.
Chakravarty, T., L. Sondergaard, J. Friedman, O. De Backer, D. Berman, K. F. Kofoed, H. Jilaihawi, T. Shiota, Y. Abramowitz, T. H. Jorgensen, T. Rami, S. Israr, G. Fontana, M. de Knegt, A. Fuchs, P. Lyden, A. Trento, D. L. Bhatt, M. B. Leon, R. R. Makkar, and Resolve and S. Investigators. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 389:2383–2392, 2017.
Chandran, K. B., S. E. Rittgers, and A. P. Yoganathan. Biofluid Mechanics: The Human Circulation. Boca Raton, FL: CRC Press, 2007.
De Marchena, E., J. Mesa, S. Pomenti, Y. K. C. Marin, X. Marincic, K. Yahagi, E. Ladich, R. Kutz, Y. Aga, M. Ragosta, A. Chawla, M. E. Ring, and R. Virmani. Thrombus formation following transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 8:728–739, 2015.
Fedorov, A., R. Beichel, J. Kalpathy-Cramer, J. Finet, J. C. Fillion-Robin, S. Pujol, C. Bauer, D. Jennings, F. Fennessy, M. Sonka, J. Buatti, S. Aylward, J. V. Miller, S. Pieper, and R. Kikinis. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 30:1323–1341, 2012.
Ferziger, J. H., and M. Peric. Computational Methods for Fluid Dynamics. Berlin: Springer, 2001.
Fuchs, A., O. De Backer, M. Brooks, M. C. de Knegt, G. Bieliauskas, M. Yamamoto, R. Yanagisawa, K. Hayashida, L. Sondergaard, and K. F. Kofoed. Subclinical leaflet thickening and stent frame geometry in self-expanding transcatheter heart valves. EuroIntervention 13:e1067–e1075, 2017.
Gunning, P. S., N. Saikrishnan, L. M. McNamara, and A. P. Yoganathan. An in vitro evaluation of the impact of eccentric deployment on transcatheter aortic valve hemodynamics. Ann. Biomed. Eng. 42:1195–1206, 2014.
Hatoum, H., J. Dollery, S. M. Lilly, J. Crestanello, and L. P. Dasi. Impact of patient-specific morphologies on sinus flow stasis in transcatheter aortic valve replacement: an in vitro study. J. Thorac. Cardiovasc. Surg. 157:540–549, 2018.
Hatoum, H., J. Dollery, S. M. Lilly, J. A. Crestanello, and L. P. Dasi. Sinus hemodynamics variation with tilted transcatheter aortic valve deployments. Ann. Biomed. Eng. 47:75–84, 2018.
Jahren, S. E., P. P. Heinisch, D. Hasler, B. M. Winkler, S. Stortecky, T. Pilgrim, M. C. Londono, T. Carrel, H. von Tengg-Kobligk, and D. Obrist. Can bioprosthetic valve thrombosis be promoted by aortic root morphology? An in vitro study. Interact. Cardiovasc. Thorac. Surg. 27:108–115, 2018.
Jilaihawi, H., F. M. Asch, E. Manasse, C. E. Ruiz, V. Jelnin, M. Kashif, H. Kawamori, Y. Maeno, Y. Kazuno, N. Takahashi, R. Olson, J. Alkhatib, D. Berman, J. Friedman, N. Gellada, T. Chakravarty, and R. R. Makkar. Systematic CT methodology for the evaluation of subclinical leaflet thrombosis. JACC Cardiovasc. Imaging 10:461–470, 2017.
Kapadia, S., E. M. Tuzcu, and L. G. Svensson. Anatomy and flow characteristics of neosinus: important consideration for thrombosis of transcatheter aortic valves. Circulation 136:1610–1612, 2017.
Kelm, M., L. Goubergrits, J. Bruening, P. Yevtushenko, J. F. Fernandes, S. H. Sundermann, F. Berger, V. Falk, T. Kuehne, S. Nordmeyer, and C. Group. Model-based therapy planning allows prediction of haemodynamic outcome after aortic valve replacement. Sci. Rep. 7:9897, 2017.
Langtry, R. B., and F. R. Menter. Correlation-based transition modeling for unstructured parallelized computational fluid dynamics codes. AIAA J. 47:2894–2906, 2009.
Mack, M. J., M. B. Leon, V. H. Thourani, R. Makkar, S. K. Kodali, M. Russo, S. R. Kapadia, S. C. Malaisrie, D. J. Cohen, P. Pibarot, J. Leipsic, R. T. Hahn, P. Blanke, M. R. Williams, J. M. McCabe, D. L. Brown, V. Babaliaros, S. Goldman, W. Y. Szeto, P. Genereux, A. Pershad, S. J. Pocock, M. C. Alu, J. G. Webb, and C. R. Smith. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 380:1695–1705, 2019.
Madukauwa-David, I. D., P. A. Midha, R. Sharma, K. McLain, R. Mitra, K. Crawford, S. H. Yoon, R. R. Makkar, and A. P. Yoganathan. Characterization of aortic root geometry in transcatheter aortic valve replacement patients. Catheter Cardiovasc. Interv. 93:134–140, 2018.
Madukauwa-David, I. D., V. Sadri, P. A. Midha, V. Babaliaros, C. Aidun, and A. P. Yoganathan. An evaluation of the influence of coronary flow on transcatheter heart valve neo-sinus flow stasis. Ann. Biomed. Eng. 48:169–180, 2019.
Makkar, R. R., G. Fontana, H. Jilaihawi, T. Chakravarty, K. F. Kofoed, O. De Backer, F. M. Asch, C. E. Ruiz, N. T. Olsen, A. Trento, J. Friedman, D. Berman, W. Cheng, M. Kashif, V. Jelnin, C. A. Kliger, H. Guo, A. D. Pichard, N. J. Weissman, S. Kapadia, E. Manasse, D. L. Bhatt, M. B. Leon, and L. Sondergaard. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N. Engl. J. Med. 373:2015–2024, 2015.
Menter, F. R., R. B. Langtry, S. R. Likki, Y. B. Suzen, P. G. Huang, and S. Völker. A correlation-based transition model using local variables—Part I: model formulation. J. Turbomach. 128:413–422, 2004.
Midha, P. A., V. Raghav, J. F. Condado, I. U. Okafor, S. Lerakis, V. H. Thourani, V. Babaliaros, and A. P. Yoganathan. Valve type, size, and deployment location affect hemodynamics in an in vitro valve-in-valve model. JACC Cardiovasc. Interv. 9:1618–1628, 2016.
Midha, P. A., V. Raghav, R. Sharma, J. F. Condado, I. U. Okafor, T. Rami, G. Kumar, V. H. Thourani, H. Jilaihawi, V. Babaliaros, R. R. Makkar, and A. P. Yoganathan. The fluid mechanics of transcatheter heart valve leaflet thrombosis in the neosinus. Circulation 136:1598–1609, 2017.
Moore, B., and L. P. Dasi. Spatio-temporal complexity of the aortic sinus vortex. Exp. Fluids 55:1770, 2014.
Pache, G., S. Schoechlin, P. Blanke, S. Dorfs, N. Jander, C. D. Arepalli, M. Gick, H. J. Buettner, J. Leipsic, M. Langer, F. J. Neumann, and P. Ruile. Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur. Heart J. 37:2263–2271, 2016.
Popma, J. J., G. M. Deeb, S. J. Yakubov, M. Mumtaz, H. Gada, D. O’Hair, T. Bajwa, J. C. Heiser, W. Merhi, N. S. Kleiman, J. Askew, P. Sorajja, J. Rovin, S. J. Chetcuti, D. H. Adams, P. S. Teirstein, G. L. Zorn, III, J. K. Forrest, D. Tchetche, J. Resar, A. Walton, N. Piazza, B. Ramlawi, N. Robinson, G. Petrossian, T. G. Gleason, J. K. Oh, M. J. Boulware, H. Qiao, A. S. Mugglin, M. J. Reardon, and I. Evolut Low Risk Trial. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N. Engl. J. Med. 380:1706–1715, 2019.
Raghav, V., C. Clifford, P. Midha, I. Okafor, B. Thurow, and A. Yoganathan. Three-dimensional extent of flow stagnation in transcatheter heart valves. J. R. Soc. Interface 16:20190063, 2019.
Raghav, V., S. Sastry, and N. Saikrishnan. Experimental assessment of flow fields associated with heart valve prostheses using particle image velocimetry (PIV): recommendations for best practices. Cardiovasc. Eng. Technol. 9:273–287, 2018.
Ruile, P., J. Minners, P. Breitbart, S. Schoechlin, M. Gick, G. Pache, F. J. Neumann, and M. Hein. Medium-term follow-up of early leaflet thrombosis after transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 11:1164–1171, 2018.
Saikrishnan, N., S. Gupta, and A. P. Yoganathan. Hemodynamics of the Boston Scientific Lotus™ valve: an in vitro study. Cardiovasc. Eng. Technol. 4:427–439, 2013.
Singh-Gryzbon, S., V. Sadri, M. Toma, E. L. Pierce, Z. A. Wei, and A. P. Yoganathan. Development of a computational method for simulating tricuspid valve dynamics. Ann. Biomed. Eng. 47:1422–1434, 2019.
Tan, F. P. P., A. Borghi, R. H. Mohiaddin, N. B. Wood, S. Thom, and X. Y. Xu. Analysis of flow patterns in a patient-specific thoracic aortic aneurysm model. Comput. Struct. 87:680–690, 2009.
Tang, G. H. L., S. Verma, and D. L. Bhatt. Transcatheter aortic valve replacement in low-risk patients. Circulation 140:801–803, 2019.
Toninato, R., J. Salmon, F. M. Susin, A. Ducci, and G. Burriesci. Physiological vortices in the sinuses of Valsalva: an in vitro approach for bio-prosthetic valves. J. Biomech. 49:2635–2643, 2016.
Trusty, P. M., V. Sadri, I. D. Madukauwa-David, E. Funnell, N. Kamioka, R. Sharma, R. Makkar, V. Babaliaros, and A. P. Yoganathan. Neosinus flow stasis correlates with thrombus volume post-TAVR: a patient-specific in vitro study. JACC Cardiovasc. Interv. 12:1288–1290, 2019.
Xu, F., S. Morganti, R. Zakerzadeh, D. Kamensky, F. Auricchio, A. Reali, T. J. R. Hughes, M. S. Sacks, and M.-C. Hsu. A framework for designing patient-specific bioprosthetic heart valves using immersogeometric fluid–structure interaction analysis. Int. J. Numer. Method Biomed. Eng. 34:e2938, 2018.
Acknowledgments
This study was supported with funds from the Wallace H. Coulter Chair Endowment and the Marvin H. and Rita Floyd Endowment. Beatrie Ncho also holds an International Fellowship from American Association of University Women. The authors acknowledge the use of ANSYS software which was provided through an Academic Partnership between ANSYS, Inc.; the Cardiovascular Fluid Mechanics Laboratory at Georgia Tech, particularly Mandy Salmon and Joe Kun Huck Choi; and Ming-Chen Hsu and Fei Xu from Iowa State University for their assistance with the parametric leaflet modeling.
Conflicts of interest
Philipp Blanke is a Consultant for Edwards Lifesciences, Tendyne, Neovasc and Circle Cardiovascular Imaging. Ajit Yoganathan is a Consultant or Researcher for St. Jude Medical, Boston Scientific, Sorin Biomedica and Edwards Lifesciences. The other authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Umberto Morbiducci oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh-Gryzbon, S., Ncho, B., Sadri, V. et al. Influence of Patient-Specific Characteristics on Transcatheter Heart Valve Neo-Sinus Flow: An In Silico Study. Ann Biomed Eng 48, 2400–2411 (2020). https://doi.org/10.1007/s10439-020-02532-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02532-x