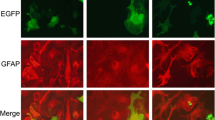
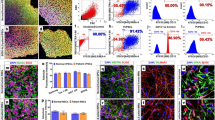
Abstract
Motor neuron degeneration in amyotrophic lateral sclerosis (ALS) caused by mutations in superoxide dismutase 1 (SOD1) is partly non-cell autonomous, involving cellular dysfunction of astrocytes. Whether non-cell autonomous effects occur in other forms of ALS, such as TAR DNA binding protein 43 (TDP-43)-related disease, remains unclear. Here, we characterised the impact of mutant TDP-43 expression on primary astrocytes derived from transgenic TDP-43A315T mice. Mutant TDP-43 astrocytes revealed evidence for TDP-43 pathology, shown by cytoplasmic TDP-43 inclusions and accumulation in insoluble cell fractions which was exacerbated by proteasomal inhibition. l-glutamate uptake, measured using an [3H]D-aspartate assay, was impaired in mutant TDP-43 astrocytes, while ATP accumulation was abnormal, suggesting mutant TDP-43 induced astrocytic dysfunction. Astrocyte activation coupled with spinal and cortical motor neuron loss in transgenic TDP-43A315T mice could imply non-cell autonomous effects of astrocytes in vivo. These data demonstrate mutant TDP-43-mediated cell autonomous effects on astrocytes that may contribute to motor neuron pathology in ALS.





Similar content being viewed by others
References
Taylor JP, Brown RH Jr, Cleveland DW (2016) Decoding ALS: from genes to mechanism. Nature 539:197–206
Ilieva H, Polymenidou M, Cleveland DW (2009) Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187:761–772
Neumann M, Sampathu D, Kwong LK, Truax AC, Micsenyi M, Chou T, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey L, Miller BL, Masliah E, Mackenzie IR, Feldman H, Kretzschmar HA, Trojanowski JQ (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science (New York, NY) 10:130–133
Geser F, Brandmeir NJ, Kwong LK, Martinez-Lage M, Elman L, McCluskey L, Xie SX, Lee VM, Trojanowski JQ (2008) Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol 65:636–641
Gregory JM, McDade K, Bak TH, Pal S, Chandran S, Smith C, Abrahams S (2019) Executive, language and fluency dysfunction are markers of localised TDP-43 cerebral pathology in non-demented ALS. J Neurol Neurosurg Psychiatry 91:149
Serio A, Bilican B, Barmada SJ, Ando DM, Zhao C, Siller R, Burr K, Haghi G, Story D, Nishimura AL, Carrasco MA, Phatnani HP, Shum C, Wilmut I, Maniatis T, Shaw CE, Finkbeiner S, Chandran S (2013) Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc Natl Acad Sci USA 110:4697–4702
Haidet-Phillips AM, Gross SK, Williams T, Tuteja A, Sherman A, Ko M, Jeong YH, Wong PC, Maragakis NJ (2013) Altered astrocytic expression of TDP-43 does not influence motor neuron survival. Exp Neurol 250:250–259
Rojas F, Cortes N, Abarzua S, Dyrda A, van Zundert B (2014) Astrocytes expressing mutant SOD1 and TDP43 trigger motoneuron death that is mediated via sodium channels and nitroxidative stress. Front Cell Neurosci 8:24
Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH (2009) TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA 106:18809–18814
Perera ND, Sheean RK, Scott JW, Kemp BE, Horne MK, Turner BJ (2014) Mutant TDP-43 deregulates AMPK activation by PP2A in ALS models. PLoS ONE 9:e90449
O'Shea RD, Lau CL, Farso MC, Diwakarla S, Zagami CJ, Svendsen BB, Feeney SJ, Callaway JK, Jones NM, Pow DV, Danbolt NC, Jarrott B, Beart PM (2006) Effects of lipopolysaccharide on glial phenotype and activity of glutamate transporters: Evidence for delayed up-regulation and redistribution of GLT-1. Neurochem Int 48:604–610
Apricò K, Beart PM, Crawford D, O’Shea RD (2004) Binding and transport of [3H](2S,4R)-4-methylglutamate, a new ligand for glutamate transporters, demonstrate labeling of EAAT1 in cultured marine astrocytes. J Neurosci Res 75:751–759
Wallis N, Lau CL, Farg MA, Atkin JD, Beart PM, O’Shea RD (2018) SOD1 mutations causing familial amyotrophic lateral sclerosis induce toxicity in astrocytes: evidence for bystander effects in a continuum of astrogliosis. Neurochem Res 43:166–179
Moldrich RX, Apricó K, Diwakarla S, O’Shea RD, Beart PM (2002) Astrocyte mGlu2/3-mediated cAMP potentiation is calcium sensitive: studies in murine neuronal and astrocyte cultures. Neuropharmacology 43:189–203
Scotter EL, Vance C, Nishimura AL, Lee YB, Chen HJ, Urwin H, Sardone V, Mitchell JC, Rogelj B, Rubinsztein DC, Shaw CE (2014) Differential roles of the ubiquitin proteasome system and autophagy in the clearance of soluble and aggregated TDP-43 species. J Cell Sci 127:1263–1278
Tong J, Huang C, Bi F, Wu Q, Huang B, Liu X, Li F, Zhou H, Xia XG (2013) Expression of ALS-linked TDP-43 mutant in astrocytes causes non-cell-autonomous motor neuron death in rats. EMBO J 32:1917
Yan S, Wang CE, Wei W, Gaertig MA, Lai L, Li S, Li XJ (2014) TDP-43 causes differential pathology in neuronal versus glial cells in the mouse brain. Hum Mol Genet 23:2678–2693
Muyderman H, Yew WP, Homkajorn B, Sims NR (2010) Astrocytic responses to DNA delivery using nucleofection. Neurochem Res 35:1771–1779
Beart PM, Chen T, Turner BJ, Muyderman H (2019) Proteomic analysis of astrocytes carrying mutant TDP-43 reveals dysfunction of ubiquitin proteasome, energetics, cytoskeletal function and anti-oxidant responses: a case for therapeutics targeting glutathione and iron homeostasis. Brain Neurosci Adv Suppl 3:PS091
Braak H, Ludolph AC, Neumann M, Ravits J, Del Tredici K (2017) Pathological TDP-43 changes in Betz cells differ from those in bulbar and spinal alpha-motoneurons in sporadic amyotrophic lateral sclerosis. Acta Neuropathol 133:79–90
Moujalled D, Grubman A, Acevedo K, Yang S, Ke YD, Moujalled DM, Duncan C, Caragounis A, Perera ND, Turner BJ, Prudencio M, Petrucelli L, Blair I, Ittner LM, Crouch PJ, Liddell JR, White AR (2017) TDP-43 mutations causing amyotrophic lateral sclerosis are associated with altered expression of RNA-binding protein hnRNP K and affect the Nrf2 antioxidant pathway. Hum Mol Genet 26:1732–1746
Khalfallah Y, Kuta R, Grasmuck C, Prat A, Durham HD, Vande Velde C (2018) TDP-43 regulation of stress granule dynamics in neurodegenerative disease-relevant cell types. Sci Rep 8:7551
Acknowledgements
PMB is pleased to contribute a paper to this Special Issue honouring Michael Robinson who has been a colleague furthering the neurochemical cause via Neurochemistry International and internationally (ISN) for some 20 years. Funding for this project was provided by the Australian NHMRC (Project Grant 1023780 P.M.B.; Fellowship 1020401 P.M.B.; Fellowship 1137024 B.J.T; and joint NHMRC-ARC Fellowship 1110040 S.K.B) and the Stafford Fox Medical Research Foundation. The Florey Institute of Neuroscience & Mental Health acknowledge Victorian Government Operational Infrastructure Support.
Author information
Authors and Affiliations
Contributions
BJT, PMB and HM conceived the study design. CLL, MDFC and DT performed the experimental procedures. SKB and BJT collated the data and wrote the manuscript and all authors approved the final version.
Corresponding author
Ethics declarations
Ethics Approval
Approved by the Florey Institute Animal Ethics Committee (permit number: 11-084).
Additional information
Special Issue In honor of Professor Michael Robinson
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2020_3048_MOESM1_ESM.tiff
Supplementary file1 (TIFF 2703 kb) Suppl Figure 1: Inhibiting the UPS alters astrocyte morphology. Untreated 12 DIV TDP-43A315T astrocytes had increased GFAP expression compared to WT astrocytes. Treating astrocytes with increasing doses of MG-132 altered astrocyte morphology and GFAP expression but there was no longer a noticeable difference between WT and TDP-43A315T astrocytes. Scale bar 50 µm.
11064_2020_3048_MOESM2_ESM.tiff
Supplementary file2 (TIFF 2703 kb) Supplemental Figure 2: Inhibiting the Ubiquitin Proteasome System (UPS) causes decreased TDP-43 in the soluble nuclear fraction in 12 DIV TDP-43A315T astrocytes. The synthetic peptide MG-132 was used at increasing doses to inhibit the UPS. MG-132 had no effect on TDP-43 levels in the nucleus in WT astrocytes and whilst TDP-43A315T astrocytes of matching doses had significantly increased TDP-43 levels compared to their WT counterparts, the 3.0 μM dose of MG-132 caused a significant decrease in the amount of TDP-43 in the nuclear protein fraction compared to untreated TDP-43A315T astrocytes (A). There was no difference in the proportion of cytoplasmic TDP-43 to total soluble TDP-43 in any groups, regardless of genotype (B) indicating re-distribution of TDP-43 to the insoluble protein fraction in TDP-43A315T astrocytes. #p<0.05 compared to untreated TDP43A315T, *p<0.05 compared to the WT treated with the same concentration. n = 3 mice per genotype for all groups.
Rights and permissions
About this article
Cite this article
Barton, S.K., Lau, C.L., Chiam, M.D.F. et al. Mutant TDP-43 Expression Triggers TDP-43 Pathology and Cell Autonomous Effects on Primary Astrocytes: Implications for Non-cell Autonomous Pathology in ALS. Neurochem Res 45, 1451–1459 (2020). https://doi.org/10.1007/s11064-020-03048-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03048-5