Abstract
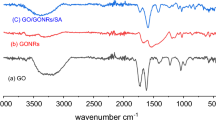
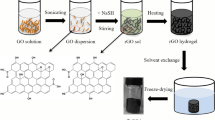
Graphene/graphene nanoribbons hybrid aerogel (GA/GNRs) was prepared via a hydrothermal process by using graphene oxide as precursor, and the graphene oxide nanoribbons unzipped from multi-walled carbon nanotubes were compound together. Various techniques were used to investigate the morphology, surface properties and structure of aerogel samples. Besides, the adsorption properties of the hybrid aerogel for the removal of U(VI) were further studied. The results showed that GNRs was grafted on the surface of graphene, and the specific surface area and porous pores structure were enhanced. The adsorption process of hybrid aerogel for U(VI) was pH-dependent, rapid, spontaneous and endothermic, and the maximum adsorption capacity for U(VI) was calculated to be 327.8 mg/g, which is expected to separate uranium from wastewater.










Similar content being viewed by others
References
Lourenço J, Marques S, Carvalho FP et al (2017) Uranium mining wastes: the use of the fish embryo acute toxicity test (FET) test to evaluate toxicity and risk of environmental discharge. Sci Total Environ 605–606:391–404. https://doi.org/10.1016/j.scitotenv.2017.06.125
Ochiai A, Imoto J, Suetake M et al (2018) Uranium dioxides and debris fragments released to the environment with cesium-rich microparticles from the Fukushima Daiichi Nuclear Power Plant. Environ Sci Technol 52:2586–2594. https://doi.org/10.1021/acs.est.7b06309
Schneider E, Carlsen B, Tavrides E et al (2013) Measures of the environmental footprint of the front end of the nuclear fuel cycle. Energy Econ 40:898–910. https://doi.org/10.1016/j.eneco.2013.01.002
Rosenberg E, Pinson G, Tsosie R et al (2016) Uranium remediation by ion exchange and sorption methods: a critical review. Johnson Matthey Technol Rev 60:59–77. https://doi.org/10.1595/205651316X690178
Dang DH, Novotnik B, Wang W et al (2016) Uranium isotope fractionation during adsorption, (co)precipitation, and biotic reduction. Environ Sci Technol 50:12695–12704.https://doi.org/10.1021/acs.est.6b01459
Mehta VS, Maillot F, Wang Z et al (2014) Effect of co-solutes on the products and solubility of uranium(VI) precipitated with phosphate. Chem Geol 364:66–75. https://doi.org/10.1016/j.chemgeo.2013.12.002
Newsome L, Morris K, Lloyd JR (2014) The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem Geol 363:164–184. https://doi.org/10.1016/j.chemgeo.2013.10.034
Kumar JR, Kim JS, Lee JY, Yoon HS (2011) A brief review on solvent extraction of uranium from acidic solutions. Sep Purif Rev 40:77–125. https://doi.org/10.1080/15422119.2010.549760
Higginbotham AL, Kosynkin DV, Sinitskii A et al (2010) Lower-defect graphene oxide nanoribbons from multiwalled carbon nanotubes. ACS Nano 4:2059–2069. https://doi.org/10.1021/nn100118m
Wu P, Wang Y, Hu X et al (2019) Synthesis of magnetic graphene oxide nanoribbons composite for the removal of Th(IV) from aqueous solutions. J Radioanal Nucl Chem 319:1111–1118. https://doi.org/10.1007/s10967-018-6375-2
Wang Y, Wang Z, Ang R et al (2015) Synthesis of amidoximated graphene oxide nanoribbons from unzipping of multiwalled carbon nanotubes for selective separation of uranium(VI). RSC Adv 5:89309–89318. https://doi.org/10.1039/c5ra15977f
Cheng C, Han H, Ren CL et al (2016) Edge effects on the characteristics of uranium diffusion on graphene and graphene nanoribbons. Chin Phys B 8:284–290. https://doi.org/10.1088/1674-1056/25/8/086301
Xiu T, Liu Z, Wang Y et al (2019) Thorium adsorption on graphene oxide nanoribbons/manganese dioxide composite material. J Radioanal Nucl Chem 319:1059–1067. https://doi.org/10.1007/s10967-019-06417-9
Wu P, Wang Y, Li Y et al (2019) Adsorption of Th(IV) from aqueous solution by the graphene oxide nanoribbons/chitosan composite material. J Radioanal Nucl Chem 322:553–559. https://doi.org/10.1007/s10967-019-06725-0
Miwa M, Nakajima A, Fujishima A et al (2000) Effects of the surface roughness on sliding angles of water droplets on superhydrophobic surfaces. Langmuir 16:5754–5760. https://doi.org/10.1021/la991660o
Bell MS, Shahraz A, Fichthorn KA, Borhan A (2015) Effects of hierarchical surface roughness on droplet contact angle. Langmuir 31:6752-6762. https://doi.org/10.1021/acs.langmuir.5b01051
Wang C, Yang S, Ma Q et al (2017) Preparation of carbon nanotubes/graphene hybrid aerogel and its application for the adsorption of organic compounds. Carbon 118:765–771. https://doi.org/10.1016/j.carbon.2017.04.001
Fan W, Wang D, Sun Z et al (2019) Graphene/graphene nanoribbon aerogels decorated with S-doped MoSe2 nanosheets as an efficient electrocatalyst for hydrogen evolution. Inorg Chem Front 6:1209–1216. https://doi.org/10.1039/c9qi00064j
Chen L, Du R, Zhang J, Yi T (2015) Density controlled oil uptake and beyond: from carbon nanotubes to graphene nanoribbon aerogels. J Mater Chem A 3:20547–20553. https://doi.org/10.1039/c5ta04370k
He YR, Li SC, Li XL et al (2018) Graphene (rGO) hydrogel: a promising material for facile removal of uranium from aqueous solution. Chem Eng J 338:333–340. https://doi.org/10.1016/j.cej.2018.01.037
Ding Y, Zhu J, Wang C et al (2016) Multifunctional three-dimensional graphene nanoribbons composite sponge. Carbon N Y 104:133–140. https://doi.org/10.1016/j.carbon.2016.03.058
Sun Z, Fan W, Liu T (2017) Graphene/graphene nanoribbon aerogels as tunable three-dimensional framework for efficient hydrogen evolution reaction. Electrochim Acta 250:91–98. https://doi.org/10.1016/j.electacta.2017.08.009
Kim KH, Oh Y, Islam MF (2012) Graphene coating makes carbon nanotube aerogels superelastic and resistant to fatigue. Nat Nanotechnol 7:562–566. https://doi.org/10.1038/nnano.2012.118
Chen L, Du R, Zhu J et al (2015) Three-dimensional nitrogen-doped graphene nanoribbons aerogel as a highly efficient catalyst for the oxygen reduction reaction. Small 11:1423–1429. https://doi.org/10.1002/smll.201402472
Wang W, Wu Y, Jiang Z et al (2018) Formation mechanism of 3D macroporous graphene aerogel in alcohol-water media under gamma-ray radiation. Appl Surf Sci 427:1144–1151. https://doi.org/10.1016/j.apsusc.2017.09.058
He Y, Li J, Li L, Li J (2016) Gamma-ray irradiation-induced reduction and self-assembly of graphene oxide into three-dimensional graphene aerogel. Mater Lett 177:76–79. https://doi.org/10.1016/j.matlet.2016.04.187
Lim MB, Hu M, Manandhar S et al (2015) Ultrafast sol-gel synthesis of graphene aerogel materials. Carbon N Y 95:616–624. https://doi.org/10.1016/j.carbon.2015.08.037
Lim J, Lee GY, Lee HJ et al (2019) Open porous graphene nanoribbon hydrogel via additive-free interfacial self-assembly: fast mass transport electrodes for high-performance biosensing and energy storage. Energy Storage Mater 16:251–258. https://doi.org/10.1016/j.ensm.2018.06.004
Ma J, Li C, Yu F, Chen J (2014) 3D single-walled carbon nanotube/graphene aerogels as Pt-free transparent counter electrodes for high efficiency dye-sensitized solar cells. Chemsuschem 7:3304–3311. https://doi.org/10.1002/cssc.201403062
Wang Z, Yue L, Liu ZT et al (2012) Functional graphene nanocomposite as an electrode for the capacitive removal of FeCl3 from water. J Mater Chem 22:14101–14107. https://doi.org/10.1039/c2jm32175k
Hyun SP, Davis JA, Sun K, Hayes KF (2012) Uranium(VI) reduction by iron(II) monosulfide mackinawite. Environ Sci Technol 46:3369–3376. https://doi.org/10.1021/es203786p
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21966005, 21601033, 21661003, 11705027, 21866006, 11875105), and East China University of Technology Graduate Innovation Fund Project (DHYC-201906), and the Natural Science Foundation of Jiangxi Province(20192BAB202007) and Key project of Jiangxi Natural Science Foundation (20192ACB21001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, X., Wang, Y., Wu, P. et al. Preparation of graphene/graphene nanoribbons hybrid aerogel and its application for the removal of uranium from aqueous solutions. J Radioanal Nucl Chem 325, 207–215 (2020). https://doi.org/10.1007/s10967-020-07208-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07208-3