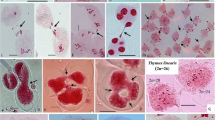
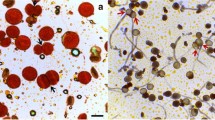
Abstract
Chromosomal behaviour during megasporogenesis and microsporogenesis has been studied in ornamental Delphinium ajacis L. Meiosis in female sex cell initiates later than male. The floral buds which carry egg mother cell (EMC) at diplotene stage has pollen mother cells (PMCs) at tetrad stage of meiosis suggesting protandry. Although the 16 chromosomes formed regular eight bivalents in both the sex cells, they differed in overall chiasma frequency which was 32.95% higher in EMCs and found to be 18.52 ± 2.12 per cell. In PMCs, the average chiasma frequency recorded was 13.93 ± 1.40 per cell. Interestingly, this variation in chiasma frequency was largely confined to the two large bivalents which shared 42.61% chiasma per EMC. The use of Q–Q plot, Box plot and Whisker plot showed departure in the chiasma frequency distributions in EMCs and PMCs from the normal distribution pattern. The difference in chiasma frequency in the two sex cells was significant at all levels as indicated by the low P values of 3.094 × 10−11 obtained from nonparametric test, i.e. Wilcoxon rank-sum test. It is suggested that the two different mechanisms of recombination are operational in the two sex cells, and the sex differences of chiasma frequency could have arisen due to differential epigenetic modifications of the chromatin which pattern the double-strand breaks, and the position and frequency of crossing over visible as chiasmata.




Similar content being viewed by others
References
Agnihotri P., Jena S. N., Husain D. and Husain T. 2014 Perspective of the genus Delphinium Linnaeus (Ranunculaceae) in India. Pleione 8, 344–352.
Armstrong S. J. and Jones G. H. 2001 Female meiosis in wild-type Arabidopsis thaliana and in two meiotic mutants. Sex. Pl. Rep. 13, 177–183.
Bennett M. D., Finch R. A., Smith J. B. and Rao M. K. 1973 The time and duration of female meiosis in wheat, rye and barley. Proc. R. Soc. London, Ser. B. 183, 301–319.
Blanché C. 1991 Revisió biosistemàtica del gènere Delphinium L. a la península Ibèrica i a les illes balears. In Arxius de la secció de ciències, vol 98, pp. 1–288. Institut d’Estudis Catalans.
Bojko M. 1985 Human meiosis IX. Crossing over and chiasma formation in oocytes. Carls. Res. Comm. 50, 43.
Brock R. D. 1954 Fertility in Lilium hybrids. Heredity 8, 409.
Choi K. 2017 Advances towards controlling meiotic recombination for plant breeding. Mol. Cell 40, 814.
Choi K., Zhao X., Lambing C., Underwood C. J., Hardcastle T. J., Serra H. et al. 2018 Nucleosomes and DNA methylation shape meiotic DSB frequency in Arabidopsis thaliana transposons and gene regulatory regions. Genome Res. 28, 532–546.
Codina-Pascual M., Campillo M., Kraus J., Speicher M. R., Egozcue J., Navarro J. et al. 2006 Crossover frequency and synaptonemal complex length: their variability and effects on human male meiosis. Mol. Hum. Reprod. 12, 123–133.
Davies E. D. G. and Jones G. H. 1974 Chiasma variation and control in pollen mother cells and embryo-sac mother cells of rye. Genet. Res. 23, 185–190.
Derreumaux S., Chaoui M., Tevanian G. and Fermandjian S. 2001 Impact of CpG methylation on structure, dynamics and solvation of cAMP DNA responsive element. Nucleic Acids Res. 29, 2314–2326.
Drouaud J., Mercier R., Chelysheva L., Bérard A., Falque M., Martin O. et al. 2007 Sex-specific crossover distributions and variations in interference level along Arabidopsis thaliana chromosome 4. PLoS Genet. 3, 106.
Fogwill M. 1958 Differences in crossing over and chromosome size in the sex cells of Lilium and Fritillaria. Chromosoma 2, 454–493.
Gohil R. N. and Kaul R. 1980 Studies on male and female meiosis in Indian Allium. I. Four diploid species. Chromosoma 77, 123–127.
Gohil R. N. and Kaul R. 1981 Studies on male and female meiosis in Indian Allium. II. Tetraploid. Allium tuberosum. Chromosoma 82, 735–739.
Gorelick R. 2003 Transposable elements suppress recombination in all meiotic eukaryotes, including automictic ancient asexuals: a reply to Schön and Martens. J. Nat. His. 37, 903–909.
Håkansson A. and Levan A. 1957 Endoduplicational meiosis in Allium odorum. Hereditas 43, 179–200.
He Y., Wang M., Dukowic-Schulze S., Zhou A., Tiang C. L., Shilo S. et al. 2017 Genomic features shaping the landscape of meiotic double-strand-break hotspots in maize. Proc. Natl. Acad. Sci. USA 114, 12231–12236.
Honda K. and Tsutsui K. 1997 Production of interspecific hybrids in the genus Delphinium via ovule culture. Euphytica 96, 331–337.
Honda K. Watanabe H. and Tsutsui K. 2003 Use of ovule culture to cross between Delphinium species of different ploidy. Euphytica 129, 275.
Iwanaga M. and Peloquin S. J. 1979 Synaptic mutant affecting only megasporogenesis in potatoes. J. Hered. 70, 385–389.
Jain H. K., Vasudevan K. N. and Basak S. L. 1963 Experimental production of a new karyotype in Delphinium. Chromosoma 14, 534–540.
Jones G. H. and Croft J. A. 1989 Chromosome pairing and chiasma formation in spermatocytes and oocytes of Dendrocoelum lactem (Turbellaria, Tricladida); a cytogenetical and ultrastructural study. Heredity 63, 97.
Kaur K. and Sidhu M. C. 2014 Meiotic studies in some medicinal angiosperms from Doaba region of Punjab, India. Int. J. Phyto. 6, 216.
Kearsey M. J., Ramsay L. D., Jenninngs D. E., Lydiate D. J. and Bohuon E. J. R. 1995 Higher recombination frequencies in female compared to male meiosis in piper. Theor. Appl. Genet. 92, 363–367.
Kelly A. L., Sharpe A. G, Nixon H., Evans E. J. and Lydiate D. J. 1997 Indistinguishable patterns of recombination resulting from male and female meioses in Brassica napus (oilseed rape). Genome 40, 49–56.
Kolar F. R., Swaroopa R. G., Nilesh V. P. and Dixit G.B. 2015 RP-HPLC analysis of an alkaloid methyllycaconitine from mutagenic Delphinium malabaricum (Huth) Munz. J. Liq. Chrom. Rel. Technol. 38, 1802–1807.
Koul K. K. and Raina S. N. 1996 Male and female meiosis in diploid and colchitetraploid Phlox drummondii Hook. (Polemoniaceae). Bot. J. Linn. Soc. 122, 243–251.
Koul K. K. and Nagpal R. 2002 Sex incidences of chiasmata variation in respect of position, distribution and frequency in some important legumes and grasses. Caryologia 55, 251–261.
Koul K. K. and Nagpal R. 2004 Male and female meiosis in Nicotiana tabacum L. Cytologia 69, 285–289.
Koul K. K., Nagpal R. and Raina S. N. 1995 Differential chromosome behavior in the male and female sex cells of Brassica oxyrrhina Coss. (Brassicaceae). Caryologia 48, 335–339.
Koul K. K., Nagpal R. and Sharma A. 2000 Chromosome behaviour in the male and female sex mother cells of wheat (Triticum aestivum L.), oat (Avena sativa L.) and pearl millet (Pennisetum americanum (L.) Leeke). Caryologia 53, 175–183.
Koul K. K., Raina S. N., Parida A. and Bisht M. S. 1999 Sex differences in meiosis between Vicia faba L. and its close wild relatives. Bot. J. Linn. Soc. 129, 239–247.
Legro R. A. H. 1961 Species hybrids in Delphinium. Euphytica 10, 1–23.
Maloisel L. and Rossignol J. L. 1998 Suppression of crossing-over by DNA methylation in Ascobolus. Genes Dev. 12, 1381–1389.
Mandal S. K. and Basu R. K. 1978 Cytology of endosperm of Delphinium ajacis. L. (Ranunculaceae) ornamental plants, India. J. Cytol. Genet. 13, 23–25.
Mehra P. N. and Remanandan P. 1972 Cytology of some W. Himalayan Ranunculaceae. Cytologia 37, 281–296.
Nelson Jr O. E. and Clary G. B. 1952 Genic control of semi-sterility in maize: an inbred with pollen semi-sterility and ovule semi-sterility caused by different genes. J. Hered. 43, 205–210.
Noda S. 1975 Achiasmate meiosis in the Fritillaria japonica group. Heredity 34, 373.
Norberg J. and Vihinen M. 2001 Molecular dynamics simulation of the effects of cytosine methylation on structure of oligonucleotides. J. Mol. Struct. Theochem. 546, 51–62.
Padmore R. C. A. O., Cao L. and Kleckner N. 1991 Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell 66, 1239–1256.
Pratap K., Kaur V., Gahlyan S., Jangra A., Pradeep A., Rani M. and Maken S. 2016 Exploring the phytochemicals of Delphinium ajacis and their applications in biocontrol activity against some plant pathogens. J. Chem. Pharma. Res. 8, 11–18.
Quevedo C. D. C., Del Cerro A. L., Santos J. L. and Jones G. H. 1997 Correlated variation of chiasma frequency and synaptonemal complex length in Locust amigratoria. Heredity 78, 515.
Royal Horticultural Society 1949 A tentative check-list of Delphinium names, pp. 1–112. London.
Sharma G. and Gohil R. N. 2011 Occurrence of differential meiotic associations and additional chromosomes in the embryo-sac mother cells of Allium roylei Stearn. J. Genet. 90, 45–49.
Singh R. N. 1991 Chromosome association and behaviour in autotetraploid Delphinium ajacis L. Cytologia 56, 479–483.
Singh R. N. and Roy S. K. 1983 Cytomorphology of autotetraploid Delphinium. Cell Chr. Res. 1, 59–64.
Subramanian D. 1985 Cytotaxonomical studies in south Indian Ranunculaceae. Cytologia 50, 759–768.
Ved Brat S. 1966 Genetic systems in Allium. II. Sex differences in meiosis. In The chromosomes today (ed. C. D. Darlington and K. R. Lewis), vol. 1, pp. 31–49. Oliver and Boyd, Edinburgh.
Vizir I. Y. and Korol A. B. 1990 Sex difference in recombination frequency in Arabidopsis. Heredity 65, 379.
Vosa C. G. 1972 Two track heredity: differentiation of male and female meiosis in Tulbaghia. Caryologia 25, 275–281.
Wallace B. M. N. and Hulten M. A. 1985 Meiotic chromosome pairing in the normal human female. Annals Hum. Genet. 49, 215–226.
Wang Y. and Copenhaver G. P. 2018 Meiotic recombination: mixing it up in plants. Ann. Rev. Plant. Biol. 69, 577–609.
Yamada S., Kim S., Tischfield S. E., Jasin M., Lange J. and Keeney S. 2017 Genomic and chromatin features shaping meiotic double-strand break formation and repair in mice. Cell Cycle 16, 1870–1884.
Yelina N. E., Choi K., Chelysheva L., Macaulay M., De Snoo, B., Wijnker E. et al. 2012 Epigenetic remodeling of meiotic crossover frequency in Arabidopsis thaliana DNA methyltransferase mutants. PLoS Genet. 8, e1002844.
Yelina N. E., Lambing C., Hardcastle T. J., Zhao X., Santos B. and Henderson I. R. 2015 DNA methylation epigenetically silences crossover hot spots and controls chromosomal domains of meiotic recombination in Arabidopsis. Genes Dev. 29, 2183–2202.
Acknowledgements
KKK thanks Sanjeev Dutt Sharma, the Librarian, Hindu College, for providing access to the journals beyond reach. The technical help rendered by Santosh Kumar and Ravi Kumar is also thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: H. A. Ranganath
Rights and permissions
About this article
Cite this article
Koul, K.K., Nagpal, R. & Nain, K. Male and female meiosis evince differential patterns in chiasma formation: a case study of ornamental plant, Delphinium ajacis L.. J Genet 99, 40 (2020). https://doi.org/10.1007/s12041-020-1179-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12041-020-1179-x