Abstract
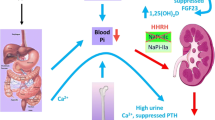
The main congenital conditions of hypophosphatemia expressed in adulthood include several forms of hereditary hypophosphatemic rickets and a congenital disorder of vitamin D metabolism characterized by osteomalacia and hypophosphatemia in adult patients. Hypophosphatemia in adults is defined as serum phosphate concentration < 0.80 mmol/L. The principal regulators of phosphate homeostasis, as is well known, are parathyroid hormone (PTH), activated vitamin D, and Fibroblast Growth Factor 23 (FGF23). Differential diagnosis of hypophosphatemia is based on the evaluation of mechanisms leading to this alteration, such as high PTH activity, inadequate phosphate absorption from the gut, or renal phosphate wasting, either due to primary tubular defects or high FGF23 levels. The most common inherited form associated to hypophosphatemia is X-linked hypophosphatemic rickets (XLH), caused by PHEX gene mutations with enhanced secretion of the FGF23. Until now, the management of hypophosphatemia in adulthood has been poorly investigated. It is widely debated whether adult patients benefit from the conventional treatments normally used for pediatric patients. The new treatment for XLH with burosumab, a recombinant human IgG1 monoclonal antibody that binds to FGF23, blocking its activity, may change the pharmacological management of adult subjects with hypophosphatemia associated to FGF23-dependent mechanisms.
Similar content being viewed by others
References
Marcucci G, Cianferotti L, Beck-Peccoz P, Capezzone M, Cetani F, Colao A, Davì MV, degli Uberti E, Del Prato S, Elisei R, Faggiano A, Ferone D, Foresta C, Fugazzola L, Ghigo E, Giacchetti G, Giorgino F, Lenzi A, Malandrino P, Mannelli M, Marcocci C, Masi L, Pacini F, Opocher G, Radicioni A, Tonacchera M, Vigneri R, Zatell MC, Brandi ML (2015) Rare diseases in clinical endocrinology: a taxonomic classification system. J Endocrinol Invest 38:193–259
Manghat P, Sodi R, Swaminathan R (2014) Phosphate homeostasis and disorders. Ann Clin Biochem 51:631–656
Marcucci G, Masi L, Ferrarì S, Haffner D, Javaid MK, Kamenický P, Reginster JY, Rizzoli R, Brandi ML (2018) Phosphate wasting disorders in adults. Osteoporos Int 29:2369–2387
McKenna MJ, Martin-Grace J, Crowley R, Twomey PJ, Kilbane MT (2019) Congenital hypophosphataemia in adults: determinants of bone turnover markers and amelioration of renal phosphate wasting following total parathyroidectomy. J Bone Miner Metab 37:685–693
Penido M, Alon US (2012) Phosphate homeostasis and its role in bone health. Pediatr Nephrol 27:2039–2048
Quarles LD (2012) Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat Rev Endocrinol 8:276–286
Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M (2009) FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Ren Physiol 297:F282–F291
Imel EA, Econs MJ (2012) Approach to the hypophosphatemic patient. J Clin Endocrinol Metab 97:696–706
Zhang X, Imel EA, Ruppe MD et al (2016) Pharmacokinetics and pharmacodynamics of a human monoclonal anti-FGF23 antibody (KRN23) in the first multiple ascending-dose trial treating adults with X-linked hypophosphatemia. J Clin Pharmacol 56:176–185
Collins M (2018) Burosumab: at long last, an effective treatmen for FGF23-associated hypophosphatemia. J Bone Miner Res 33:1381–1382
Carpenter TO, Imel EA, Ruppe MD, Weber TJ, Klausner MA, Wooddell MM, Kawakami T, Ito T, Zhang X, Humphrey J, Insogna KL, Peacock M (2014) Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Investig 124:1587–1597
Imeal EA, Zhang X, Ruppe MD, Weber TJ, Klausner MA, Ito T, Vergeire M, Humphrey JS, Glorieux FH, Portale AA, Insogna K, Peacock M, Carpenter TO (2015) Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab 100:2565–2573
Ruppe MD, Zhang X, Imel EA, Weber TJ, Klausner MA, Ito T, Vergeire M, Humphrey JS, Glorieux FH, Portale AA, Insogna K, Peacock M, Carpenter TO (2016) Effect of four monthly doses of a human monoclonal anti-FGF23 antibody (KRN23) on quality of life in X-linked hypophosphatemia. Bone Rep 5:158–162
Insogna KL, Briot K, Imel EA, Kamenický P, Ruppe MD, Portale AA, Weber T, Pitukcheewanont P, Cheong HI, Jan de Beur S, Imanishi Y, Ito N, Lachmann RH, Tanaka H, Perwad F, Zhang L, Chen CY, Theodore-Oklota C, Mealiffe M, San Martin J, Carpenter TO (2018) A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res 33:1383–1393
Bastepe M, Juppner H (2008) Inherited hypophosphatemic disorders in children and the evolving mechanisms of phosphate regulation. Rev Endocr Metab Disord 9:171–180
Beck-Nielsen SS, Brock-Jacobsen B, Gram J, Brixen K, Jensen TK (2009) Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol 160:491–497
Endo I, Fukumoto S, Ozono K, Namba N, Inoue D, Okazaki R, Yamauchi M, Sugimoto T, Minagawa M, Michigami T, Nagai M, Matsumoto T et al (2015) Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: prevalence, biochemical data and treatment. Endocr J 62:811–816
Rafaelsen S, Johansson S, Ræder H, Bjerknes R (2016) Hereditary hypophosphatemia in Norway: a retrospective population- based study of genotypes, phenotypes, and treatment complications. Eur J Endocrinol 174:125–136
Carpenter TO (2012) The expanding family of hypophosphatemic syndromes. J Bone Miner Metab 30:1–9
David V, Martin A, Hedge AM, Drezner MK, Rowe PS (2011) ASARM peptides: PHEX-dependent and -independent regulation of serum phosphate. Am J Physiol Renal Physiol 300:F783–F791
Chesher D, Oddy M, Darbar U, Sayal P, Casey A, Ryan A, Sechi A, Simister C, Waters A, Wedatilake Y, Lachmann RH, Murphy E (2018) Outcome of adult patients with X- linked hypophosphatemia caused by PHEX gene mutations. J Inherit Metab Dis 41:865–976
Whyte MP, Schranck FW, Armamento-Villareal R (1996) X- Linked hypophosphatemia: a search for gender, race, anticipation, or parent of origin effects on disease expression in children. J Clin Endocrinol Metab 81:4075–4080
Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD (2006) Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab 291:E38–E49
Barros NM, Hoac B, Neves RL, Addison WN, Assis DM, Murshed M, Carmona AK, McKee MD (2013) Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia. J Bone Min Res 28:688–699
Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ (2010) Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab 95:1846–1850
Bai X, Miao D, Xiao S, Qiu D, St-Arnaud R, Petkovich M, Gupta A, Goltzman D, Karaplis AC (2016) CYP24 inhibition as a therapeutic target in FGF23-mediated renal phosphate wasting disorders. J Clin Invest 126:667–680
Beck-Nielsen SS, Brusgaard K, Rasmussen LM, Brixen K, Brock-Jacobsen B, Poulsen MR, Vestergaard P, Ralston SH, Albagha OM, Poulsen S, Haubek D, Gjørup H, Hintze H, Andersen MG, Heickendorff L, Hjelmborg J, Gram J (2010) Phenotype presentation of hypophosphatemic rickets in adults. Calcif Tissue Int 87:108–119
Che H, Roux C, Etcheto A, Rothenbuhler A, Kamenicky P, Linglart A, Briot K (2016) Impaired quality of life in adults with X- linked hypophosphatemia and skeletal symptoms. Eur J Endocrinol 174:325–333
Biosse Duplan M, Coyac BR, Bardet C, Zadikian C, Rothenbuhler A, Kamenicky P, Briot K, Linglart A, Chaussain C (2017) Phosphate and vitamin D prevent periodontitis in X- linked hypophosphatemia. J Dent Res 96:388–395
Verge CF, Lam A, Simpson JM, Cowell CT, Howard NJ, Silink M (1991) Effects of therapy in X- linked hypophosphatemic rickets. N Engl J Med 325:1843–1848
Berndt M, Ehrich JH, Lazovic D, Zimmermann J, Hillmann G, Kayser C, Prokop M, Schirg E, Siegert B, Wolff G, Brodehl J (1996) Clinical course of hypophosphatemic rickets in 23 adults. Clin Nephrol 45:33–41
Linglart A, Biosse-Duplan M, Briot K, Chaussain C, Esterle L, Guillaume-Czitrom S, Kamenicky P, Nevoux J, Prié D, Rothenbuhler A, Wicart P, Harvengt P (2014) Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr Connect 3:R13–R30
Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL (2011) A clinician’s guide to X- linked hypophosphatemia. J Bone Miner Res 26:1381–1388
Beck-Nielsen SS, Mughal Z, Haffner D, Nilsson O, Levtchenko E, Ariceta G, de Lucas CC, Schnabel D, Jandhyala R, Mäkitie O (2019) FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis 14(1):58
Murali SK, Andrukhova O, Clinkenbeard EL, White KE, Erben RG (2016) Excessive osteocytic Fgf23 secretion contributes to pyrophosphate accumulation and mineralization defect in Hyp Mice. PLoS Biol 14:e1002427
Xiao ZS, Crenshaw M, Guo R, Nesbitt T, Drezner MK, Quarles LD (1998) Intrinsic mineralization defect in Hyp mouse osteoblasts. Am J Physiol 275(4 Pt 1):E700–E708
Bai X, Miao D, Xiao S, Qiu D, St-Arnaud R, Petkovich M et al (2016) CYP24 inhibition as a therapeutic target in FGF23-mediated renal phosphate wasting disorders. J Clin Invest 126:667–680
Addison WN, Azari F, Sorensen ES, Kaartinen MT, McKee MD (2007) Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up- regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem 282:15872–15883
Addison WN, Masica DL, Gray JJ, McKee MD (2010) Phosphorylation-dependent inhibition of mineralization by osteopontin ASARM peptides is regulated by PHEX cleavage. J Bone Min Res 25:695–705
Staines KA, MacRae VE, Farquharson C (2012) The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J Endocrinol 214:241–255
Yuan Q, Jiang Y, Zhao X, Sato T, Densmore M, Schüler C et al (2014) Increased osteopontin contributes to inhibition of bone mineralization in FGF23- deficient mice. J Bone Miner Res Off J Am Soc Bone Miner Res 29:693–704
Andrukhova O, Smorodchenko A, Egerbacher M, Streicher C, Zeitz U, Goetz R et al (2014) FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J 33:229–246
Aono Y, Yamazaki Y, Yasutake J, Kawata T, Hasegawa H, Urakawa I et al (2009) Therapeutic effects of Anti-FGF23 antibodies in hypophosphatemic rickets/ osteomalacia. J Bone Miner Res 24:1879–1888
Haffner D, Leifheit-Nestler M (2017) Extrarenal effects of FGF23. Pediatr Nephrol 32:753–765
Sato C, Iso Y, Mizukami T, Otabe K, Sasai M, Kurata M et al (2016) Fibroblast growth factor-23 induces cellular senescence in human mesenchymal stem cells from skeletal muscle. Biochem Biophys Res Commun 470:657–662
Connor J, Olear EA, Insogna KL, Katz L, Baker S, Kaur R et al (2015) Conventional therapy in adults With X-Linked hypophosphatemia: effects on enthesopathy and dental disease. J Clin Endocrinol Metab 100:3625–3632
Pavone V, Testa G, Gioitta Iachino S, Evola FR, Avondo S, Sessav G (2015) Hypophosphatemic rickets: etiology, clinical features and treatment. Eur J Orthop Surg Traumatol 25:221–226
Carpenter TO, Olear EA, Zhang JH, Ellis BK, Simpson CA, Cheng D, Gundberg CM, Insogna KL (2014) Effect of paricalcitol on circulating parathyroid hormone in X- linked hypophosphatemia: a randomized, double- blind, placebo- controlled study. J Clin Endocrinol Metab 99:3103–3111
Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V et al (2007) The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117:4003–4008
Mace ML, Gravesen E, Nordholm A, Olgaard K, Lewin E (2018) Fibroblast growth factor (FGF) 23 regulates the plasma levels of parathyroid hormone in vivo through the FGF receptor in normocalcemia, but not in hypocalcemia. Calcif Tissue Int 102:85–92
Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S (2002) Increased circulatory level of biologically active full- length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87:4957–4960
Carpenter TO, Insogna KL, Zhang JH, Ellis B, Nieman S, Simpson C, Olear E, Gundberg CM (2010) Circulating levels of soluble klotho and FGF23 in X- linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid status. J Clin Endocrinol Metab 95:E352–E357
Haffner D, Emma F, Eastwood DM, Duplan MB, Bacchetta J, Schnabel D, Wicart P, Bockenhauer D, Santos F, Levtchenko E, Harvengt P, Kirchhoff M, Di Rocco F, Chaussain C, Brandi ML, Savendahl L, Briot K, Kamenicky P, Rejnmark L, Linglart A (2019) Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol 15:435–455
Alon US, Monzavi R, Lilien M, Rasoulpour M, Geffner ME, Yadin O (2003) Hypertension in hypophosphatemic rickets—role of secondary hyperparathyroidism. Pediatr Nephrol 18:155–158
Nakamura Y, Takagi M, Takeda R, Miyai K, Hasegawa Y (2017) Hypertension is a characteristic complication of X- linked hypophosphatemia. Endocr J 64:283–289
Schmitt CP, Mehls O (2004) The enigma of hyperparathyroidism in hypophosphatemic rickets. Pediatr Nephrol 19:473–477
Econs MJ, McEnery PT (1997) Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab 82:674–681
Imel EA, Biggin A, Schindeler A, Munns CF (2019) FGF23, Hypophosphatemia, and Emerging Treatments. JBMR Plus 13(3):e10190
Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ (2011) Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab 96:3541–3549
Bai XY, Miao D, Goltzman D, Karaplis AC (2003) The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem 278:9843–9849
Huang X, Jiang Y, Xia W (2013) FGF23 and phosphate wasting disorders. Bone 28:120–132
Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R (2010) Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet 86:273–278
Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Höhne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nürnberg P (2003) Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet 34:379–381
Gattineni J, Baum M (2010) Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr Nephrol 25:591–601
Alon US (2011) Clinical practice. Fibroblast growth factor (FGF)23: a new hormone. Eur J Pediatr 170:545–554
Jaureguiberry G, Carpenter TO, Forman S, Harald Jüppner H, Bergwitz C (2008) A novel missense mutation in SLC34A3 that causes hereditary hypophosphatemic rickets with hypercalciuria in humans identifies threonine 137 as an important determinant of sodium-phosphate cotransport in NaPi-IIc. Am J Physiol Renal Physiol 295:F371–F379
Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM (2006) Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78:193–201
Bergwitz C, Miyamoto KI (2019) Hereditary hypophosphatemic rickets with hypercalciuria: pathophysiology, clinical presentation, diagnosis and therapy. Pflugers Arch 471:149–163
Haito-Sugino S, Ito M, Ohi A, Shiozaki Y, Kangawa N, Nishiyama T, Aranami F, Sasaki S, Mori A, Kido S, Tatsumi S, Segawa H, Miyamoto KI (2012) Processing and stability of type IIc sodium-dependent phosphate cotransporter mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria. Am J Physiol Cell Physiol 302:C1316–C1330
Devuyst O, Thakker RV (2010) Dent’s disease. Orphanet J Rare 5:28
Wrong OM, Norden AG, Feest TG (1994) Dent’s disease; a familial proximal renal tubular syndrome with low-molecular- weight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. QJM 87:473–493
Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP (2008) A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci U S A 4(105):3455–3460
Malloy PJ, Feldman D (2010) Genetic disorders and defects in vitamin D action. Endocrinol Metab Clin N Am 39:333–346
Tiosano D, Gepstein V (2012) Vitamin D action: lessons learned from hereditary 1,25-dihydroxyvitamin-D-resistant rickets patients. Curr Opin Endocrinol Diabetes Obes 19:452–459
Isojima T, Ishizawa M, Yoshimura K, Tamura M, Hirose S, Makishima M, Kitanaka S (2015) Hereditary 1,25-dihydroxyvitamin D-resistant rickets (HVDRR) caused by a VDR mutation: a novel mechanism of dominant inheritance. Bone Rep 7(2):68–73
Kitanaka S, Takeyama K, Murayama A, Sato T, Okumura K, Nogami M, Hasegawa Y, Niimi H, Yanagisawa J, Tanaka T, Kato S (1998) Inactivating mutations in the 25-hydroxyvitamin D3 1alpha-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N Engl J Med 338:653–661
Goldsweig BK, Carpenter TO (2015) Hypophosphatemic rickets: lessons from disrupted FGF23 control of phosphorus homeostasis. Curr Osteoporos Rep 13:88–97
Tiosano D, Hochberg Z (2009) Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab 27:392–401
Endo I, Fukumoto S, Ozono K, Namba N, Tanaka H, Inoue D, Minagawa M, Sugimoto T, Yamauchi M, Michigami T, Matsumoto T (2008) Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemicpatients: proposal of diagnostic criteria using FGF23 measurement. Bone 42:1235–1239
Igaki JM, Yamada M, Yamazaki Y, Koto S, Izawa M, Ariyasu D, Suzuki E, Hasegawa H, Hasegawa Y (2011) High iFGF23 level despite hypophosphatemia is one of the clinical indicators to make diagnosis of XLH. Endocr J 58:647–655
SouberbiellePrié JCD, Piketty ML, Rothenbuhler A, Delanaye P, Chanson P, Cavalier E (2017) Evaluation of a new fully automated assay for plasma intact FGF23. Calcif Tissue Int 101:510–518
Saraff V, Nadar R, Högler W (2020) New developments in the treatment of X-linked hypophosphataemia: implications for clinical management. Paediatr Drugs. 22(2):113–121
Lecoq AL, Brandi ML, Linglart A, Kamenický P (2020) Management of X-linked hypophosphatemia in adults. Metabolism 103S:154049
Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H et al (2008) Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res Off J Am Soc Bone Miner Res 23:1509–1518
Whyte MP, Carpenter TO, Gottesman GS et al (2019) Efficacy and safety of burosumab in children aged 1–4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol 7:189–199
Carpenter TO, Whyte MP, Imel EA et al (2018) Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med 378(21):1987–1998
US Food & Drug Administration. CRYSVITA (prescribing information) (2018) FDA.gov
European Medicines Agency. Crysvita. Annex I — summary of product characteristics. EMA (2018)
Imel EA, Glorieux FH, Whyte MP, Munns CF, Ward LM, Nilsson O, Simmons JH, Padidela R, Namba N, Cheong HI, Pitukcheewanont P, Sochett E, Högler W, Muroya K, Tanaka H, Gottesman GS, Biggin A, Perwad F, Mao M, Chen CY, Skrinar A, San Martin J, Portale AA (2019) Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet 15(393):2416–2427
Portale AA, Carpenter TO, Brandi ML, Briot K, Cheong HI, Cohen-Solal M, Crowley R, Jan De Beur S, Eastell R, Imanishi Y, Imel EA, Ing S, Ito N, Javaid M, Kamenicky P, Keen R, Kubota T, Lachmann R, Perwad F, Pitukcheewanont P, Ralston SH, Takeuchi Y, Tanaka H, Weber TJ, Yoo HW, Zhang L, Theodore-Oklota C, Mealiffe M, San Martin J, Insogna K (2019) Continued Beneficial Effects of Burosumab in Adults with X-Linked Hypophosphatemia: Results from a 24-Week Treatment Continuation Period After a 24-Week Double-Blind Placebo-Controlled Period. Calcif Tissue Int 105(3):271–284
Lambert AS, Zhukouskaya V, Rothenbuhler A, Linglart A (2019) X-linked hypophosphatemia: Management and treatment prospects. Joint Bone Spine 86:731–738
Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, Garringer HJ, Vidal R, Chan RJ, Goodwin CB, Hui SL, Peacock M, White KE (2011) Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A 108:E1146–E1155
Imel EA, Liu Z, Coffman M, Acton D, Econs MJ (2018) Oral Iron Therapy Normalizes Fibroblast Growth Factor 23 (FGF23) in Patients with Autosomal Dominant Hypophosphatemic Rickets. J Bone Miner Res 33:S1–S56
Tieder M, Modai D, Samuel R, Arie R, Halabe A, Bab I, Gabizon D, Liberman UA (1985) Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med 312:611–617
Tieder M, Modai D, Shaked U, Samuel R, Arie R, Halabe A, Maor J, Weissgarten J, Averbukh Z, Cohen N et al (1987) “Idiopathic” hypercalciuria and hereditary hypophosphatemic rickets. Two phenotypical expressions of a common genetic defect. N Engl J Med 316:125–129
Reginato AJ, Coquia JA (2003) Musculoskeletalmanifestations of osteomalacia and rickets. Best Pract Res Clin Rheumatol 17:1063–1080
Kremke B, Bergwitz C, Ahrens W, Schutt S, Schumacher M, Wagner V, Holterhus PM, Jüppner H, Hiort O (2009) Hypophosphatemic rickets with hypercalciuria due to mutation in SLC34A3/NaPi-IIc can be masked by vitamin D deficiency and can be associated with renal calcifications. Exp Clin Endocrinol Diabetes 117:49–56
HYP-Consortium (1995) A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet 11:130–136
ADHR Consortium (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348
Karim Z, Gerard B, Bakouh N, Alili R, Leroy C, Beck L, Silve C, Planelles G, Urena-Torres P, Grandchamp B, Friedlander G, Prie D (2008) NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med 359:1128–1135
Prie D, Huart V, Bakouh N, Planelles G, Dellis O, Gerard B, Hulin P, Benque-Blanchet F, Silve C, Grandchamp B, Friedlander G (2002) Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodiumphosphate cotransporter. N Engl J Med 347:983–991
Ehlayel AM, Copelovitch L (2019) Update on Dent Disease. Pediatr Clin North Am 66:169–178
Author information
Authors and Affiliations
Contributions
GM contributed to conceptualization of the study, performed the literature research and wrote the article. ML Brandi contributed to conceptualization of the study. All authors critically revised the article for intellectual content and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
GM declares that she has no conflict of interests. MLB has received honoraria from Amgen, Bruno Farmaceutici, Calcilytix, Kyowa Kirin; academic grants from and/or was speaker for Abiogen, Alexion, Amgen, Bruno Farmaceutici, Eli Lilly, Kyowa Kirin, MSD, NPS, Servier, Shire, SPA; and has been consultant for Alexion, Bruno Farmaceutici, Kyowa Kirin, Servier, Shire.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marcucci, G., Brandi, M.L. Congenital Conditions of Hypophosphatemia Expressed in Adults. Calcif Tissue Int 108, 91–103 (2021). https://doi.org/10.1007/s00223-020-00695-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00695-2