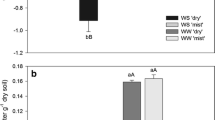
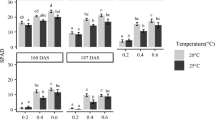
Abstract
To evaluate the transition from traditional shading cultivation to mist cultivation, a field experiment was carried out. The results demonstrated that compared with traditional shading, the mist treatment significantly reduced leaf temperature. Likewise, the higher transpiration rate also contributes to reducing leaf temperature and protects ginger from heat stress in summer. Moreover, a higher instantaneous efficiency of water use suggested that water lost via transpiration was beneficial under a mist culture system. The higher instantaneous efficiency of water use in the mist treatment was caused mainly by the higher net photosynthetic rate, which is further reflected by the higher rhizome yield of ginger under the mist culture system. Instead of lowering the temperature by lowering photon flux density, mist treatment does not seriously reduce the photon flux density while reducing the temperature of the blade. Hence, the net photosynthetic rate in the shading treatment is significantly lower than that in the mist treatment, although the maximal quantum yield of photosystem II and the actual photochemical efficiency of photosystem II in ginger in the shading treatment were significantly higher than those in the mist treatment. Lower superoxide anion, hydrogen peroxide, and malondialdehyde contents were also found after mist treatment. Lower ammonium avoids the potential risk of ammonium toxicity and is based on higher nitrate reductase, glutamine synthetase, and glutamate synthase activity but lower glutamate dehydrogenase activity. Therefore, the mist cultivation system improved the physiological characteristics and yields of ginger and can be suggested as an alternative, sustainable, and cleaner cultivation measure.










Similar content being viewed by others
Abbreviations
- CaCl2 :
-
Calcium chloride
- CAT:
-
Catalase
- Ci:
-
Intercellular CO2 concentration
- CK:
-
No shading and no mist treatment
- DTT:
-
DL-Dithiothreitol
- DW:
-
Dry weight
- E:
-
Transpiration rate
- FeCl3 :
-
Ferric chloride
- Fm:
-
Maximum fluorescence
- Fm':
-
Maximum fluorescence under light adaptation
- Fo:
-
Minimal fluorescence
- Fo':
-
Basal fluorescence after far-red illumination
- Fs:
-
Steady-state fluorescence
- Fv/Fm:
-
Maximal photochemical efficiency of PSII
- FW:
-
Fresh weight
- GDH:
-
Glutamate dehydrogenase
- GOGAT:
-
Glutamate synthase
- gs:
-
Stomatal conductance
- GS:
-
Glutamine synthetase
- H2O2 :
-
Hydrogen peroxide
- HCl:
-
Hydrochloride
- KNO3 :
-
Potassium nitrate
- LWC:
-
Leaf relative water content
- LWP:
-
Leaf water potential
- M:
-
Mist-only treatment
- MDA:
-
Malondialdehyde
- MgSO4 :
-
Magnesium sulfate
- NADH:
-
Nicotinamide adenine dinucleotide
- NBT:
-
Nitroblue tetrazolium
- NH4+ :
-
Ammonium
- NH4Cl:
-
Ammonium chloride
- NO3− :
-
Nitrate
- NPQ:
-
Non-photochemical quenching
- NR:
-
Nitrate reductase
- O2-. :
-
Superoxide anion
- PFD:
-
Photon flux density
- Pn:
-
Net photosynthetic rate
- POD:
-
Peroxidase
- PVP:
-
Polyvinyl pyrrolidone
- qP:
-
Photochemical quenching
- RWC:
-
Relative water content
- S:
-
Shading-only treatment
- S+M:
-
Shading plus mist treatment
- SD:
-
Standard deviation
- SFW:
-
Saturated fresh weight
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- TiCl4 :
-
Titanium tetrachloride
- Tris-HCl:
-
Tris(hydroxymethyl)aminomethane hydrochloride
- WUEi:
-
The instantaneous efficiency of water use
- ΦPSII:
-
Actual photochemical efficiency
References
Aliniaeifard S, Malcolm Matamoros P, van Meeteren U (2014) Stomatal malfunctioning under low VPD conditions: induced by alterations in stomatal morphology and leaf anatomy or in the ABA signaling. Physiol Plant 152(4):6888–6699
Aliniaeifard S, Van Meeteren U (2016) Stomatal characteristics and desiccation response of leaves of cut chrysanthemum (Chrysanthemum morifolium) flowers grown at high air humidity. Sci Hortic 205:84–89
Barros TC, de Mello PR, Roque CG, Arf MV, Vilela RG (2019) Silicon and salicylic acid in the physiology and yield of cotton. J Plant Nutr 42(5):458–465
Buckley TN (2019) How do stomata respond to water status? New Phytol 224:21–36
Carvalho DRA, Vasconcelos MW, Lee S, Vreugdenhil D, Hevvelink E, Carvalho SMP (2017) Moderate salinity improves stomatal functioning in rose plants grown at high relative air humidity. Environ Exp Bot 143:1–19
Cren M, Hirel B (1999) Glutamine synthetase in higher plant: regulation of gene and protein expression from the organ to the cell. Plant Cell Physiol 40(12):1187–1193
Damour G, Simonneau T, Cochard H, Urban L (2010) An overview of models of stomatal conductance at the leaf level. Plant Cell Environ 33(9):1419–1438
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1982) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32(1):93–101
Duan N, Wang J, Liu F, Chen HL, Sun F, Xu J (2018) Research progress on drought resistance of plant. Mol Plant Breed 16(15):5093–5099 (in Chinese)
Fanourakis D, Heuvelink E, Carvalho SM (2013) A comprehensive analysis of the physiological and anatomical components involved in higher water loss rates after leaf development at high humidity. J Plant Physiol 170(10):890–898
Jarvis PG, McNaughton KG (1986) Stomatal control of transpiration: scaling up from leaf to region[M]advances in ecological research, vol 15. Academic Press, pp 1–49
Krom MD (1980) Spectrophotometric determination of ammonia: a study of a modified Berthelot reaction using salicylate and dichloroisocyanurate. Analyst 105(1249):305–316
Omra RG (1980) Peroxide levels and the activities of catalase, peroxidase and indoleacetic acid oxidize during and after chilling cucumber seedlings. Plant Physiol 65:407–408
Patterson BD, MacRae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139(2):487–492
Peak D, Mott KA (2011) A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant Cell Environ 34(1):162–178
Pipitsunthonsan P, Sopharat J, Sirisuk P, Chongcheawchamnan M (2018) Leaf sensor for stomata transpiration monitoring using temperature and humidity. In: In 2018 21st international symposium on wireless personal multimedia communications, 252–255
Rao KP, Rains DW (1976) Nitrate absorption by barley: II. Influence of nitrate reductase activity. Plant Physiol 57(1):59–62
Rauckman EJ, Rosen GM, Kitchell BB (1979) Superoxide radical as an intermediate in the oxidation of hydroxylamines by mixed function amine oxidase. Mol Pharmacol 15(1):131–137
Saradadevi R, Palta JA, Khm S (2017) ABA-mediated stomatal response in regulating Wwater use during the development of terminal drought in wheat. Front Plant Sci 8:1–14
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46(3):209–221
Simonin KA, Burns E, Choat B, Barbour MM, Dawson TE, Franks PJ (2014) Increasing leaf hydraulic conductance with transpiration rate minimizes the water potential drawdown from stem to leaf. J Exp Bot 66(5):1303–1315
Skopelitis DS, Paranychianakis NV, Paschalidis KA, Pliakonis ED, Delis ID, Yakoumakis DI, Kouvarakis A, Papadakis AK, Stephanou EG, Roubelakis-Angelakis KA (2006) Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 18(10):2767–2781
Steinmetz Z, Wollmann C, Schaefer M, Buchmann C, David J, Tröger J, Muñoz K, Frör O, Schaumann GE (2016) Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci Total Environ 550:690–705
Tay AC, Abdullah AM, Awang M, Furukawa A (2007) Midday depression of photosynthesis in Enkleia malaccensis, a woody climber in a tropical rainforest. Photosynthetica 45(2):189–193
Torre S, Fjeld T, Gislerød HR, Moe R (2003) Leaf anatomy and stomatal morphology of greenhouse roses grown at moderate or high air humidity. J Am Soc Hortic Sci 128(4):598–602
Wang Y, Wei XL (2010) Advance on the Effects of different light environments on growth, physiological biochemistry and morphostructure of plant. J MT Agric Biol 29(4):353–359 (in Chinese)
Wu Z, Ren H, McGrath SP, Wu P, Zhao FJ (2011) Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol 157(1):498–508
Xia J, Yin FM, Xu K, Cao BL, (2019) Effects of fogging cultivation on microclimate in ginger field and ginger growth. Chin Veg 1(3): 41-46 (in Chinese)
Xu K (1999) Effects of ground covering grass on microclimate and ginger growth in ginger field. Chin Veg 1(2):15–17 (in Chinese)
Xu D, Gao X, Gao T, Mou J, Li J, Bu H, Zhang R, Li Q (2018) Interactive effects of nitrogen and silicon addition on growth of five common plant species and structure of plant community in alpine meadow. Catena 169:80–89
Yabuki K, Miyagawa H (1970) Studies on the effect of wind speed upon the photosynthesis. J Agric Meteoro 26(3):137–141
Yan CR, He WQ, Neil C (2014) Plastic-film mulch in Chinese agriculture: importance and problems. World Agr 4(2):32–36
Zhang LP, Jing Q, Dai TB, Jiang D, Cao WX (2008) Effects of temperature and illumination on flag leaf photosynthetic characteristics and senescence of wheat cultivars with different grain quality. JAppl Ecol 19(2):311–316 (in Chinese)
Zhao DW, Chen LP (1988) Study on the light-requirement characteristics and rational population density of ginger. Chin Veg 1(1):5–8 (in Chinese)
Zhao SJ, Liu HS, Dong XC (2002) Experimental guidance of plant physiology[M]. China Agricultural Science and Technology Press, Beijing (in Chinese)
Funding
This project was funded by the Agriculture Research System of China (Grant No. CARS-24-A-09); the Taishan Industrial Experts Programme, China (Grant No. tscy20190105), the Key Research and Development Project of Shandong Province (Grant No. 2019GNC106057); and Shandong Province’s dual-class discipline construction project, China (Grant No. SYL2017YSTD06).
Author information
Authors and Affiliations
Contributions
Bili Cao, Xie Jie, Lv Yueqiang, and Chen Zijing carried out the field studies. Bili Cao carried out the statistic studies and wrote the manuscript. Kun Xu designed the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Néstor Carrillo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, B., Xia, J., Lv, Y. et al. Effect of a mist culture system on photosynthesis and nitrogen metabolism in ginger. Protoplasma 257, 1359–1371 (2020). https://doi.org/10.1007/s00709-020-01511-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01511-2