Introduction
The bustards of Asia are one of the most threatened groups of birds worldwide (Collar et al. Reference Collar, Baral, Batbayer, Bhardwaj, Brahma, Burnside, Choudhury, Combreau, Dolman, Donald, Dutta, Gadhavi, Gore, Goroshko, Hong, Jather, Jha, Jhala, Koshkin, Lahkar, Liu, Mahood, Morales, Narwade, Natsagdorj, Nefedov, Silva, Thakuri, Wang, Zhang and Kessler2017). Of the six species recorded in the region, all are considered globally threatened or ‘Near Threatened’, two are ‘Critically Endangered’ and one ‘Endangered’ (BirdLife International 2018). Of the two ‘Critically Endangered’ species, the Bengal Florican Houbaropsis bengalensis is polytypic, with H. b. bengalensis confined to protected areas in floodplain and alluvial grasslands in north-east India and Nepal and H. b. blandini now restricted to the Tonle Sap floodplain of Cambodia, although it formerly also occurred in the Mekong Delta (Collar et al. Reference Collar, Garcia and Sharpe2018). Rice has been cultivated in the Tonle Sap floodplain for at least 1,000 years (Vanna Reference Vanna2002). Between the 9th and 15th centuries, rice cultivation was sufficiently intensive that a surplus was produced to feed the workers building Angkor Wat, the world’s largest religious building (Campbell et al. Reference Campbell, Poole, Giesen and Valbo-Jorgensen2006). This rich-ethno cultural landscape was characterised by cultivation of deep-water rice varieties, which were grown during the flood season on fields that are left fallow when floodwaters recede, coupled with patches of grassland maintained through traditional burning and grazing regimes (Gray et al. Reference Gray, Chamnan, Borey, Collar and Dolman2007). These traditional agricultural practices inadvertently replicated the processes of long extinct ungulate populations, creating ideal habitat for Bengal Florican (Gray et al. Reference Gray, Chamnan, Borey, Collar and Dolman2007). Like all bustards, Bengal Floricans are terrestrial, and require large expanses of relatively flat, open habitat to enable them to spot predators, and so that their courtship displays can be seen from great distance (Collar et al. Reference Collar, Garcia and Sharpe2018). Females nest on the ground in patches of denser vegetation, and after breeding both sexes migrate up to 60 km annually to escape rising floodwaters (Gray et al. Reference Gray, Chamnan, Collar and Dolman2009a; Packman Reference Packman2011). The Bengal Florican has little cultural significance to the people of Cambodia (Goes Reference Goes2013), but for at least a millennium its fate has been closely tied to agricultural practices of the communities living in the Tonle Sap floodplain, the rice bowl of Cambodia.
Following decades of conflict, Cambodia’s Bengal Florican population was rediscovered in 1999, and market-driven hunting was quickly identified as the most significant threat (Goes Reference Goes2013). This was addressed through rapid conservation action, focusing on raising awareness of communities and law enforcement personnel (Davidson Reference Davidson2004). As hunting pressure was reduced, conversion of grassland and low intensity agriculture to intensive dry-season rice rapidly emerged as a new and highly pertinent threat (Packman et al. Reference Packman, Gray, Collar, Evans, Van Zalinge, Virak, Lovett and Dolman2013a). Grassland cover in the Tonle Sap floodplain declined from 3,349 km2 to 1,817 km2 between 1995 and 2005, and grassland loss has subsequently continued owing to a massive and sustained expansion in dry-season rice production driven by expansion of irrigation infrastructure (Packman et al. Reference Packman, Gray, Collar, Evans, Van Zalinge, Virak, Lovett and Dolman2013a). As well as causing direct loss of Bengal Florican habitat in the outer floodplain, construction of irrigation infrastructure physically blocked the seasonal migration routes used by cattle herders, leading to a 23% increase in scrub cover at the expense of the rich inner floodplain grassland between 1995 and 2005 (Packman et al. Reference Packman, Gray, Collar, Evans, Van Zalinge, Virak, Lovett and Dolman2013a). Irrigation and concomitant mechanisation have enabled farmers to convert almost all remaining areas of grassland to agriculture and switch from cultivating deep-water rice to dry-season rice. Initially, irrigation infrastructure was constructed to service concessions granted to wealthy businessmen (Gray Reference Gray2008). However, even close to sites that retained Bengal Florican, by 2005 dry-season rice was cultivated by one third of villages, and by 2012 it was ubiquitous (Ibbett et al. Reference Ibbett, Lay, Phlai, Song, Hong, Mahood and Milner-Gulland2017). Farmers prefer the taste of deep-water rice and consider it healthier than modern varieties, but yields are tied to the extent and duration of the flood, which they perceive as becoming increasingly variable (Ibbett et al. Reference Ibbett, Lay, Phlai, Song, Hong, Mahood and Milner-Gulland2017). Irrigation allows farmers to control water levels, and the switch to dry-season rice is indeed associated with an increase in household income, although farmers do not like the heavy chemical use associated with dry-season rice and rarely keep it for home consumption (Ibbett et al. Reference Ibbett, Lay, Phlai, Song, Hong, Mahood and Milner-Gulland2017).
Sites that supported large Bengal Florican populations in the early 2000s, such as Sankor and Koup Prea Bueng Trea (see Figure 1 for locations of sites), were converted to dry-season rice relatively early in the 21st century. The limited scale of early irrigation infrastructure permitted only a single crop of dry-season rice per year, which was harvested early in the breeding cycle of the Bengal Florican (WCS unpublished data). Although Bengal Floricans typically avoid dry-season rice cultivation (Gray et al. Reference Gray, Chamnan, Collar and Dolman2009a), fallow fields and vegetated head-ponds that dried out after one crop of dry-season rice make suitable (although potentially sub-optimal) breeding habitat for Bengal Florican (Son Virak pers. obs.). Recent improvements to irrigation infrastructure mean that these areas can now support two or even three crops of dry-season rice each year, so they and the head ponds that feed them remain flooded until much later in the breeding cycle of Bengal Florican (Ibbett et al. Reference Ibbett, Lay, Phlai, Song, Hong, Mahood and Milner-Gulland2017). Since Bengal Floricans nest on the ground, this change in agricultural practices reduces or even eliminates the amount of time when there is habitat suitable for nesting or for the rearing of chicks.
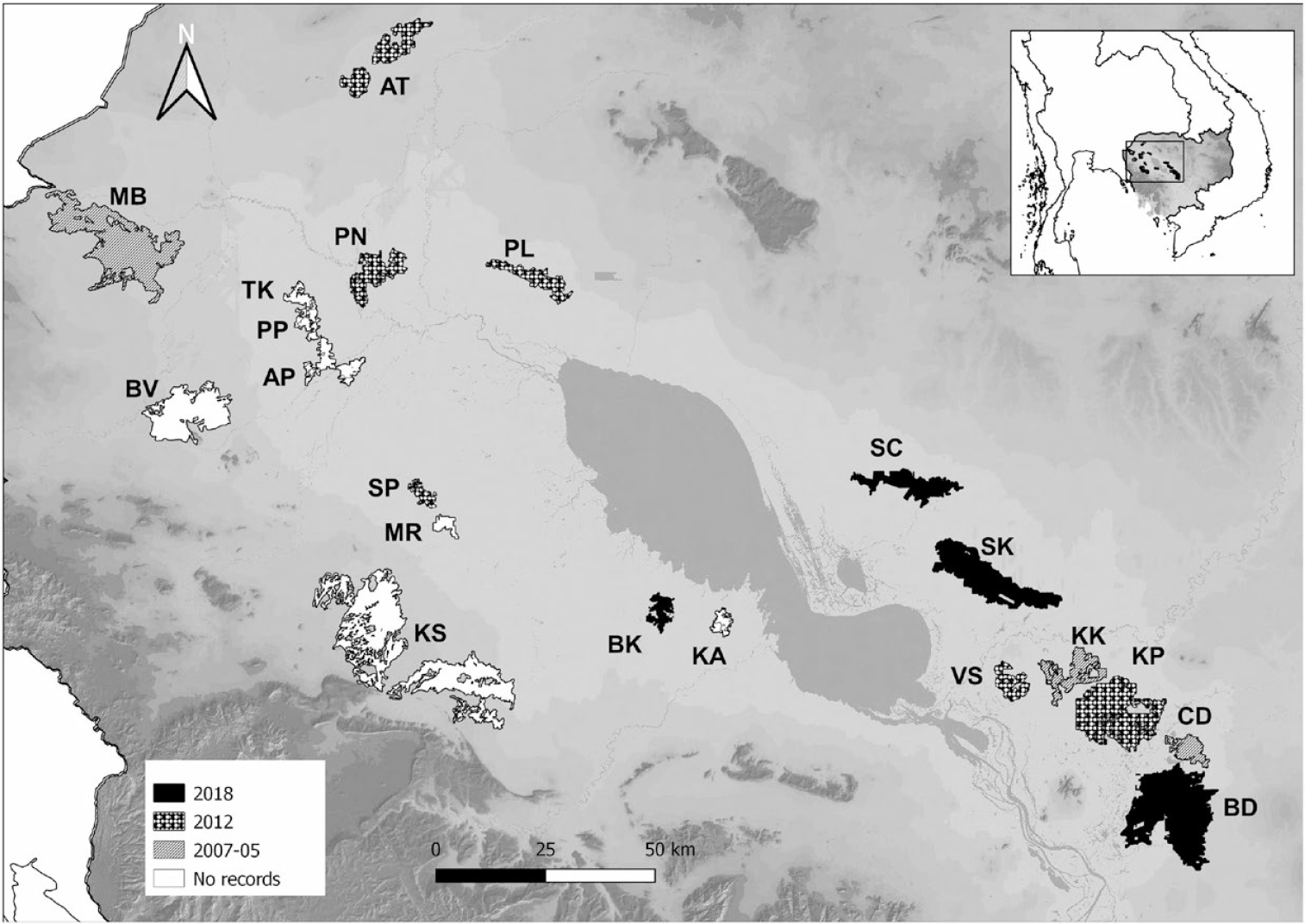
Figure 1. Map of the Tonle Sap floodplain showing sites where Bengal Florican monitoring has been conducted since 2005–2007 (see Table 1 for site codes). Shading indicates most recent census year when each site supported displaying males.
In response to the loss of much of the grassland, the first grassland reserves (Integrated Farming and Biodiversity Areas: IFBAs), totalling 349 km2, were established under provincial declarations in 2007 with the aim of preserving favourable agricultural systems (Gray et al. Reference Gray, Chamnan, Collar and Dolman2009a). Legal protection of these sites was improved in 2010 under a decree from the Ministry of Agriculture Forestry and Fisheries that established Bengal Florican Conservation Areas (BFCAs) (Packman et al. Reference Packman, Showler, Collar, Virak, Mahood, Handschuh, Evans, Chamnan and Dolman2013b). However this came at a cost because the area of floodplain breeding habitat under protection was reduced to 173 km2 (four sites constituting two management units), although an additional 138 km2 of non-breeding habitat outside the floodplain (two sites) was added to the protected area network. By this time there were already large rice concessions within Chikraeng and Chong Duong BFCAs which could not be cancelled. Although there was an agreement that these would at least not be expanded, this commitment has not been adhered to (Mahood et al. Reference Mahood, Son, Chamnan and Evans2012). In 2016 the six BFCAs were combined into one management jurisdiction under a prime ministerial sub-decree, the highest level of legal protection, which renamed them the Northern Tonle Sap Protected Landscape (for ease of reference the individual sites are still referred to as BFCAs). Field conservation of Bengal Floricans by Wildlife Conservation Society (WCS) in collaboration with the Ministry of Environment has focussed on community-based management on Stoung-Chikraeng BFCA for historical, ecological and logistical reasons.
In Cambodia a complete census of all areas that supported suitable Bengal Florican habitat in 2005 was conducted between 2005 and 2007, yielding an estimate of 416 (95% CI 333–502) displaying males (Gray et al. Reference Gray, Collar, Davidson, Dolman, Evans, Fox, Chamnan, Borey, Hout and Van Zalinge2009b). The census was repeated in 2012, when a total of 216 (95% CI 156–275) displaying males was estimated at 10 sites (Packman et al. Reference Packman, Showler, Collar, Virak, Mahood, Handschuh, Evans, Chamnan and Dolman2013b). Owing to an absence of information to the contrary the sex ratio was assumed to be equal and the total Cambodian population was estimated to be 432 (95% CI 312–550) individuals (Packman et al. Reference Packman, Showler, Collar, Virak, Mahood, Handschuh, Evans, Chamnan and Dolman2013b). After correcting for differences in survey methodology, the estimated population decline in Cambodia between the two censuses was 44–64%. H. b. blandini was predicted to be extinct within 10 years i.e. by 2023 (Packman et al. Reference Packman, Showler, Collar, Virak, Mahood, Handschuh, Evans, Chamnan and Dolman2013b). Given this dire prediction, we consider it important to provide an up-to-date assessment of the status of the total Bengal Florican population in Cambodia at the mid-point of that period, to test whether management of the habitat has been effective in stemming declines. We have also analysed population and habitat data collected over the past five years to help understand recent population trends and make conservation recommendations for the Bengal Florican and other imperilled Asian bustards.
Methods
The Bengal Florican is extremely cryptic except during the breeding season, when males perform display flights and strut around with puffed out neck feathers (Collar et al. Reference Collar, Garcia and Sharpe2018). Bengal Florican has an exploded lek breeding system; > 4 males are required for a lek to function (Gray et al. Reference Gray, Chamnan, Borey, Collar and Dolman2007) and each male needs up to 2.5 km2 of habitat from which to display (Gray et al. Reference Gray, Chamnan, Collar and Dolman2009a; Packman Reference Packman2011), so conservatively only sites >10 km2 in 2005 were considered for survey (Gray et al. Reference Gray, Collar, Davidson, Dolman, Evans, Fox, Chamnan, Borey, Hout and Van Zalinge2009b). Locations of the sites are shown in Figure 1, and sizes in Table 1. Of the 20 sites visited in 2012 (19 in 2005–2007), only 13 were visited in 2018 (Table 1). The six sites not visited in 2018 comprised four sites where no Bengal Florican was recorded during either previous survey (i.e. there have been no records since pre-2005), as well as two former breeding sites located outside of the Tonle Sap floodplain: Mongkol Borei where no bird was recorded during 2013 or in subsequent casual visits, and Ang Trapeang Thmor where there has been just one record since 2012 despite intensive survey effort. It was assumed that floricans no longer use these sites, or that if they do so, populations are likely to be too low for a functioning lek to persist. Six sites in the south-east of the Tonle Sap floodplain were also surveyed in most years between the 2012 and 2018 censuses, although no sites were surveyed in 2015 due to logistical reasons (Table 1).
Table 1. Survey sites, their area, and frequency of survey.
Numbers of displaying male Bengal Florican have been monitored almost annually in the floodplain BFCAs and the other four sites located in the south-east of the Tonle Sap floodplain. In 2012 these supported 79% of the Cambodian population (Packman et al. Reference Packman, Showler, Collar, Virak, Mahood, Handschuh, Evans, Chamnan and Dolman2013b). Annual monitoring and the 2018 total population census followed the same methods used in 2012, as detailed in Packman et al. (Reference Packman, Showler, Collar, Virak, Mahood, Handschuh, Evans, Chamnan and Dolman2013b). They are summarised here. Population estimates at sites were derived from sampling using 1-km2 replicate squares, each separated by 1 km, laid in a grid over each site. Surveys aimed to sample a minimum of 20% of the area of each site with a minimum of eight survey squares per site. Within each square, the number of displaying male Bengal Floricans was recorded over two separate one-hour periods by two different observers during the period of peak male display activity (first and last two hours of the day). Visits were conducted between mid-March and mid-May, the period of peak Bengal Florican display activity. During the 2018 census, 162 survey squares were visited twice with the maximum of the two counts of displaying males used for population estimation. An additional 40 survey squares could not be visited because they were either flooded or filled with dense scrub; their Bengal Florican population was assumed to be zero.
For each site, mean density of displaying males was calculated by dividing the maximum count of displaying males in each square by the total number of squares. This number was then multiplied by the area of the site to give a population estimate. In calculating the overall population estimate, sites were treated as strata to account for unequal survey effort among sites following (Gray et al. Reference Gray, Collar, Davidson, Dolman, Evans, Fox, Chamnan, Borey, Hout and Van Zalinge2009b) such that population estimates from sites were summed, whilst confidence intervals were calculated as the square root of the sum of site level variance. Because we used exactly the same survey method as (Packman et al. Reference Packman, Showler, Collar, Virak, Mahood, Handschuh, Evans, Chamnan and Dolman2013b), we were able to evaluate differences between population estimates in 2012 and 2018 using the Z-test; analysis was conducted in R (R Core Team 2018). The number of displaying males in a given survey square was typically 1 or 0, so we used an occupancy framework to estimate detectability, comparing the numbers of Bengal Floricans located during just the first or just the second visit to the maximum count recorded across both visits.
From 2013 onwards, area of habitat was visually estimated in visited survey squares in the following broad categories: grassland, rice, scrub, water and other. There was a total of 1,639 square/site/year habitat data points. These data were used to calculate area of grassland within each survey square in each year that it was visited, and mean grassland cover within squares was multiplied by the area of the site to estimate grassland cover within the site. We conducted linear regressions with site as a fixed factor to examine the relationship between site-level Bengal Florican populations and grassland loss and we conducted logistic regression to evaluate impacts of habitat change on Bengal Florican presence/absence in survey squares. All analyses were conducted using R (R Core Team 2018).
During the breeding season male Bengal Floricans flush easily, whilst females sit tight and flush only at very short range. To investigate the sex ratio of Bengal Floricans we conducted surveys during the non-breeding season, when they are more likely to be equally cryptic. During the non-breeding season when the floodplain is inundated, the Bengal Floricans migrate to degraded deciduous forest up to 60 km beyond the breeding grounds (Packman Reference Packman2011, Hillard Reference Hillard2012). Males and females are cryptic when not breeding, so birds were flushed by a line of three or four observers walking through the habitat, with each person separated by 10-20 m. Any Bengal Floricans flushed by the team were sexed, and the distance measured from the observers to the location where the bird had flushed. A t-test was used to evaluate whether there was a difference between flushing distances of male and female Bengal Floricans.
Results
The total population of Bengal Florican in Cambodia in 2018 was estimated as 104 (89–117) displaying males (Figure 2). This indicates that the population has declined by 52% since 2012 when the total number of displaying males was estimated as 216 (156–275) (Packman et al. Reference Packman, Showler, Collar, Virak, Mahood, Handschuh, Evans, Chamnan and Dolman2013b). The rate of decline has not changed since 2005–2012. Detectability during the 2018 survey was extremely high, single visits recorded 81% of the maximum number of displaying males detected over two visits, so the population estimate is probably accurate. Although females are more cryptic than displaying males during the breeding season, data gathered during the non-breeding season found no statistically significant difference in flushing distance between the sexes (one-sample t (85) = -1.714, P = 0.090), although two males flushed at a much greater distance than any of the females (Figure 3). Of the 88 Bengal Floricans recorded between 2011 and 2017 during non-breeding season surveys, a ratio of three males to one female was observed. This ratio indicates that there may only be 34 (30–39) females remaining in Cambodia. The total population (excluding non-displaying males) is therefore estimated at 138 (119–156).
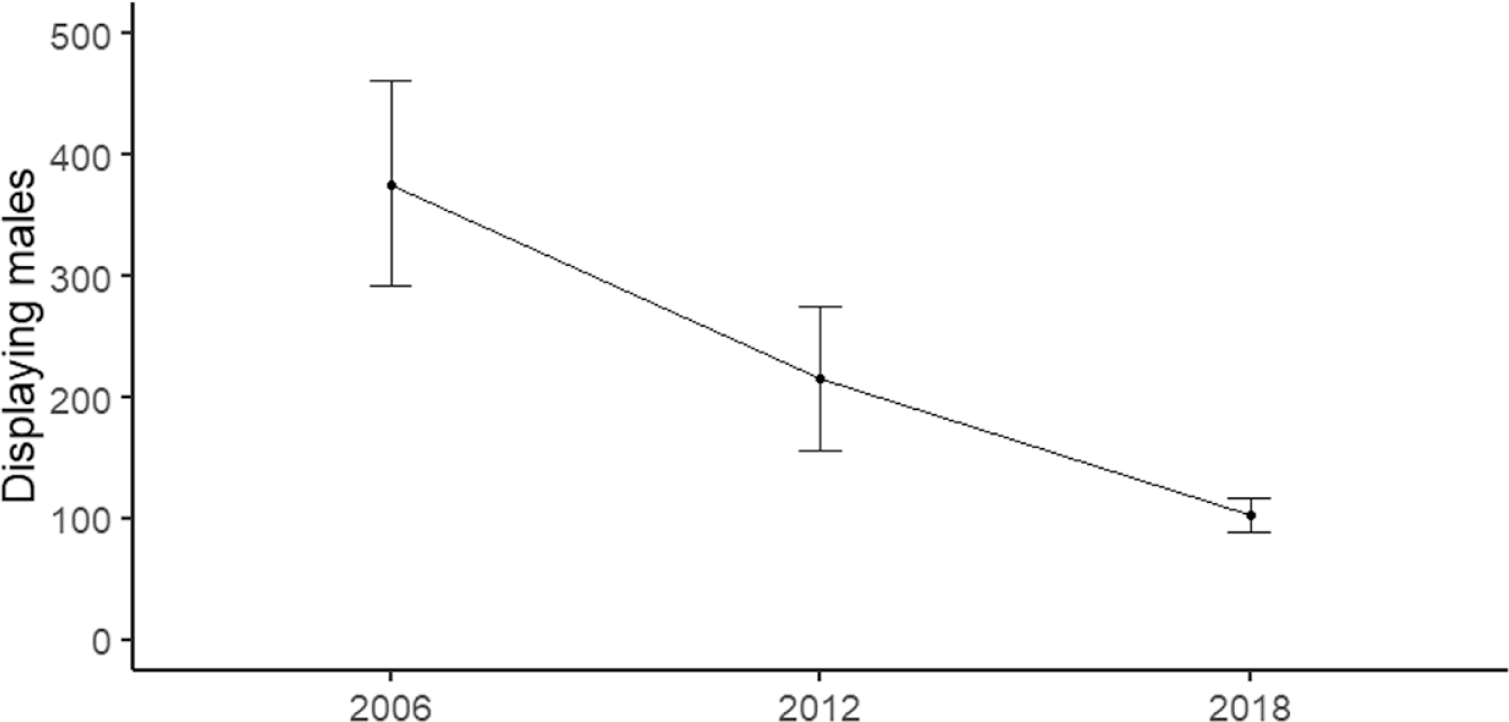
Figure 2. Estimate of population of displaying male Bengal Floricans in Cambodia
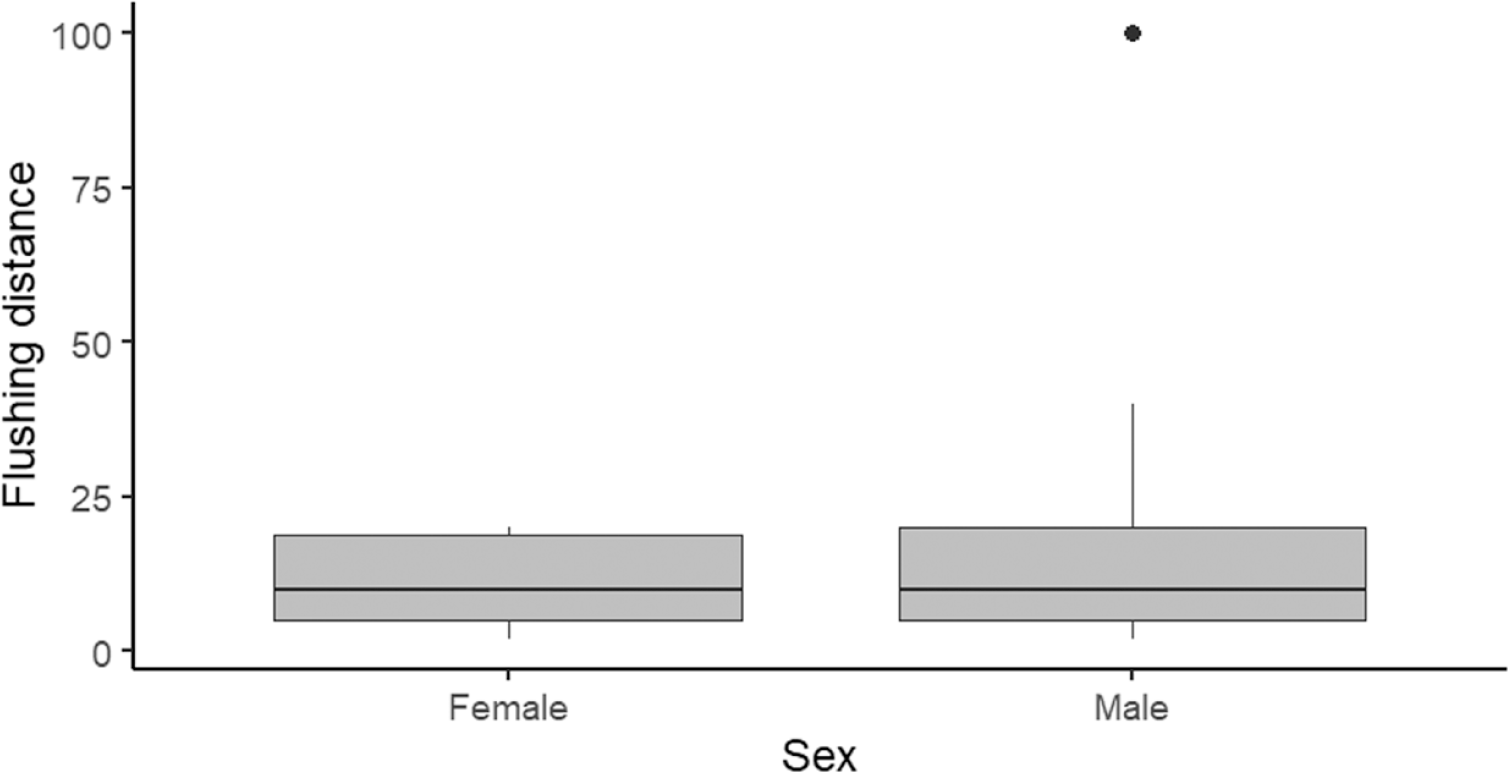
Figure 3. Flushing distance of Bengal Florican in the non-breeding season (n = 88, Females = 22, Males = 66).
Displaying male Bengal Floricans were detected in only four sites during the 2018 census compared to 10 in 2012 and 11 in 2005–2007 (Table 2). A small number of non-displaying male Bengal Floricans were recorded at one additional site (Koup Prea Bueng Trea). Bengal Floricans have now been extirpated from all except one of the sites located outside of their core range in the south-east of the Tonle Sap floodplain, and that site, Bakan, located in the south-west of the floodplain, is estimated to support only 3 (0–11) displaying males. Of the other sites that still support displaying male Bengal Florican in 2018, nearly half the birds were found in one relatively small protected area where the population is stable: Stoung-Chikraeng BFCA. Baray District (a relatively large site encompassing Baray BFCA and unprotected grassland to the east) now supports one third of the total population, although population trends there are unclear and the change between 2012 and 2018 was non-significant (Figure 4). The population at Sankor, a large unprotected site that supported the largest population in 2005–2007 (87; 54–120), underwent a statistically significant decline between 2012 and 2018 to just 21 (0–44) displaying males (Z = 1.66, P = 0.048; Figure 4).
Table 2. Estimated numbers of displaying males by site (with 95% CIs) for censuses conducted in 2005, 2006 or 2007 (Gray et al. Reference Gray, Collar, Davidson, Dolman, Evans, Fox, Chamnan, Borey, Hout and Van Zalinge2009b), 2012 (Packman et al. Reference Packman, Showler, Collar, Virak, Mahood, Handschuh, Evans, Chamnan and Dolman2013b) and 2018; % population change (2012–2018) with significance (Z-test: **p<0.005, *p<0.05, NSp>0.05).
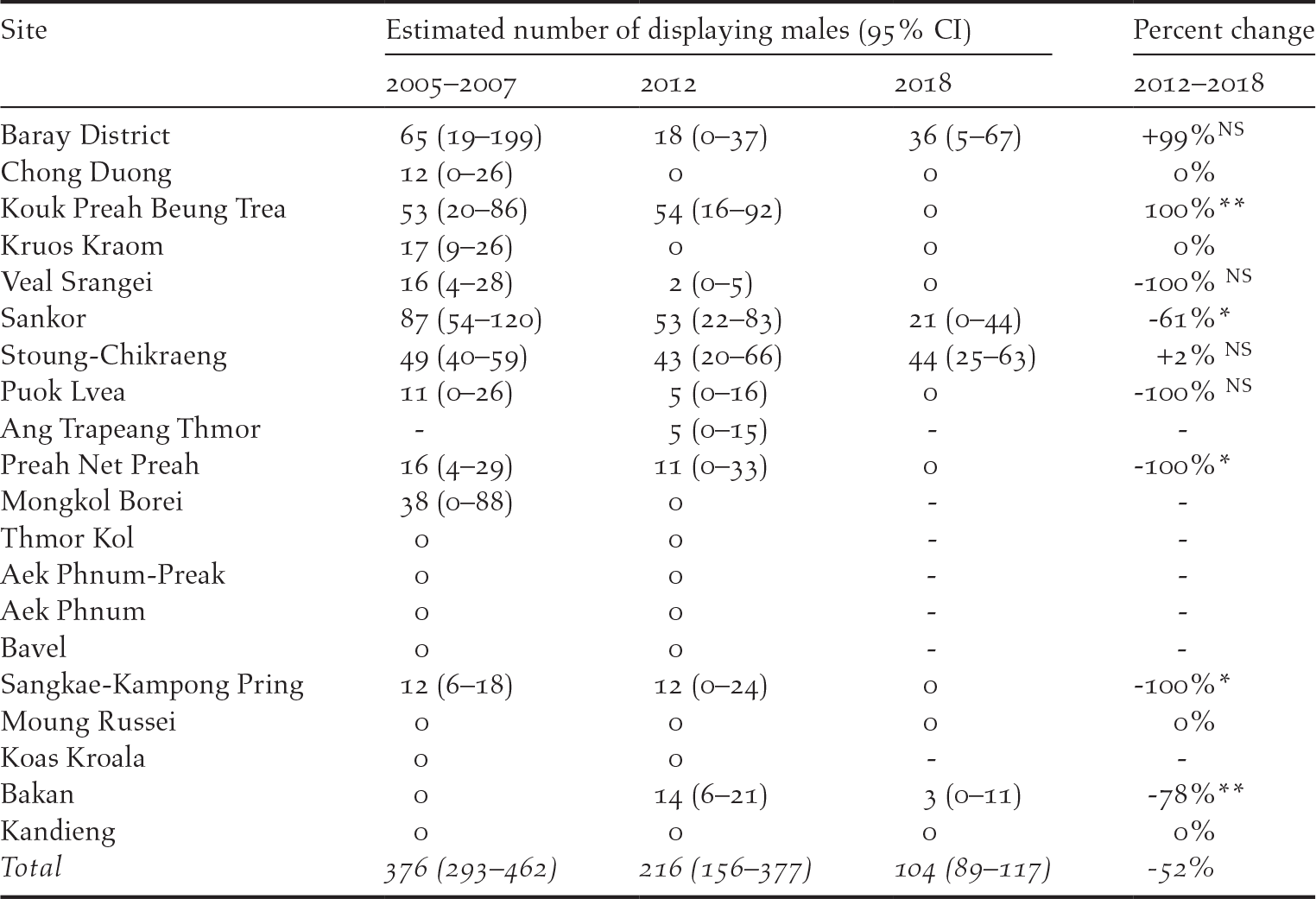
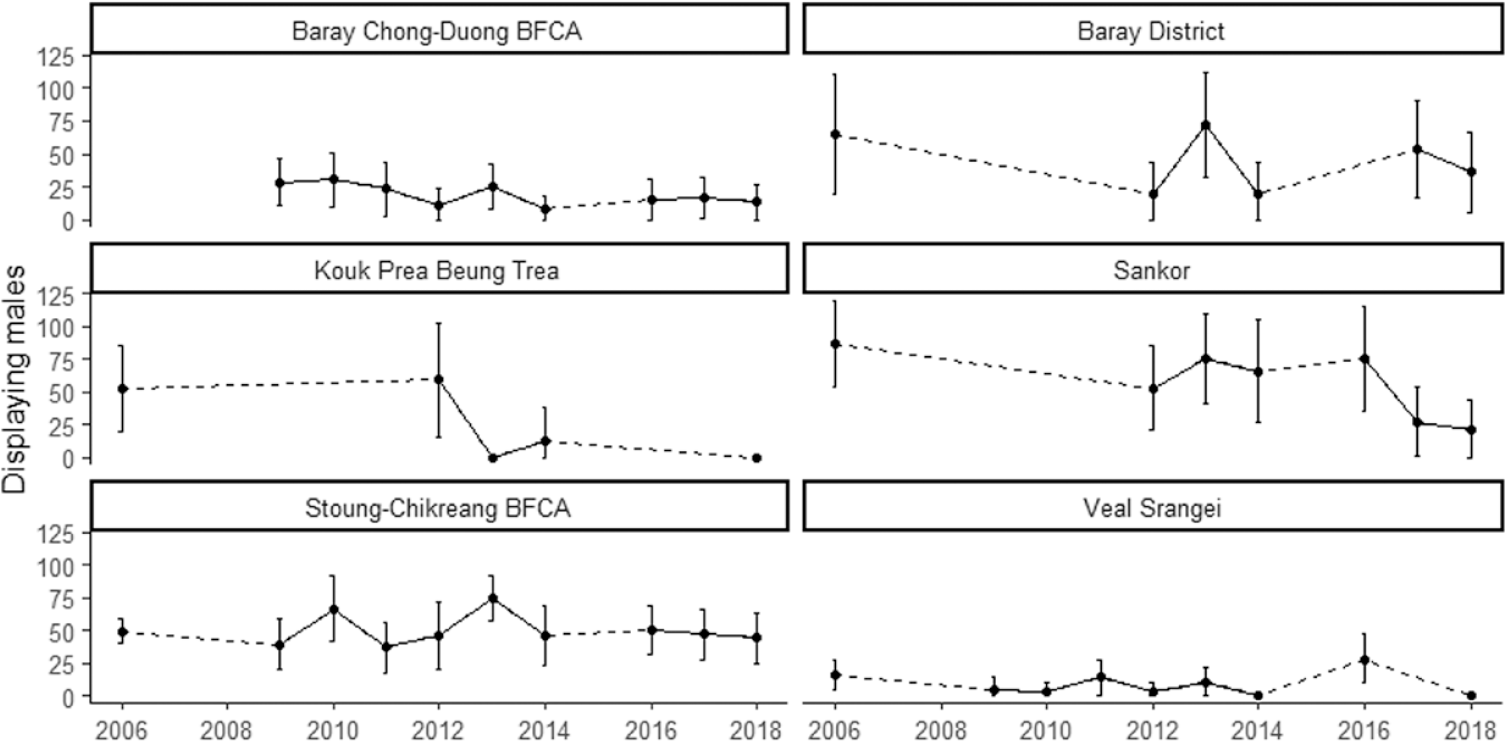
Figure 4. Site-specific trends in displaying male Bengal Floricans in the core Cambodian breeding range (dashed lines bridge years with no population census).
Small sites historically supported the highest densities of Bengal Florican (Gray et al. Reference Gray, Collar, Davidson, Dolman, Evans, Fox, Chamnan, Borey, Hout and Van Zalinge2009b). In 2018, relatively small sites continue to hold the highest densities of displaying male Bengal Floricans, and as the total population has declined, the relative importance of small sites has increased. Nonetheless, most of the sites with the highest density of floricans in 2012 now support none (e.g. Sangkae Kampong Pring). Numbers at larger sites with lower densities have also declined rapidly (e.g. Sankor), or been lost entirely over the last six years (Table 2).
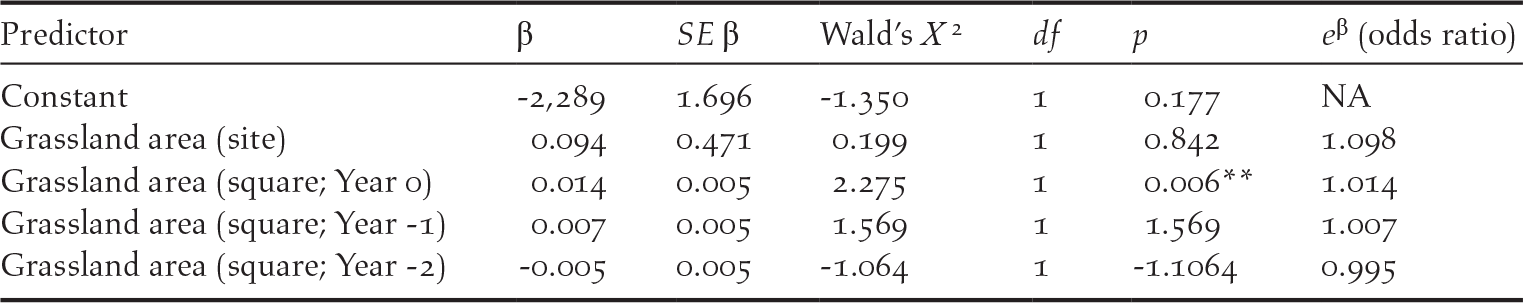
Near-annual monitoring of Bengal Florican and habitat in the south-eastern subset of sites revealed that the area of grassland in a site in the previous year has a stronger influence on population size than area of grassland in the year of the population census (Y 0: F (6, 19) = 9.15, P < 0.001, R 2 = 0.66; Y -1: F (6, 5) = 11.82, P < 0.008, R 2 = 0.86). Moreover, when there was less than 25 km2 of grassland at a site, displaying males were no longer recorded (Figure 5). The one exception to this is the data from Sankor in 2018, where a population of 21 (0–44) displaying males was estimated (Table 2). However the area of grassland in Sankor was still high in 2017 (57 km2), declining catastrophically to 9.6 km2 in 2018 when the birds may simply have been returning to areas that had been grassland in the previous year. The presence or absence of a displaying male Bengal Florican within a given survey square was not influenced by the area of grassland within the site where the square was located (Table 3). However, male Bengal Floricans were more likely to display from survey squares that contained a greater area of grassland, although the probability of a male displaying in a square was unrelated to the amount of grassland in previous years (Table 3).

Figure 5. Impact of grassland loss on number of male Bengal Florican in a site.
Table 3. Logistic regression of Bengal Florican occurrence in survey squares between 2012 and 2018 in the southeast floodplain (data from different years and sites are pooled)
Discussion
In 2013 it was predicted that the Bengal Florican could be extinct in Cambodia within 10 years, based on the rate of decline between 2005–2007 and 2012 (Packman et al. Reference Packman, Showler, Collar, Virak, Mahood, Handschuh, Evans, Chamnan and Dolman2013b). Five years later the results of the 2018 census indicate that the rate of decline has remained constant, meaning the prognosis for the population is still dire. Results affirm the notion that grassland loss caused by agricultural intensification is the greatest threat to Cambodia’s Bengal Florican population. Our data confirm that males return to display in territories that supported suitable habitat in the previous breeding season (Packman Reference Packman2011), but that they will not continue to display in squares that no longer contain suitable habitat for attracting a mate. This indicates a degree of home-range plasticity that was suspected but not previously confirmed. It offers hope that birds dispersing from habitat that has been destroyed may colonise new sites if suitable habitat is created, and they could potentially use seasonal fallows within a rotational farming system. Our results suggest a minimum threshold grassland area of approximately 25 km2 for persistence of a functioning exploded lek of Bengal Florican (Figure 5), a significantly larger area than was previously suspected (Gray et al. Reference Gray, Collar, Davidson, Dolman, Evans, Fox, Chamnan, Borey, Hout and Van Zalinge2009b). Satellite telemetry has indicated that, although male floricans display within a relatively small area (1.6 ± 0.3 to 2.6 ± 0.9 km2), they use an area at least 10 times larger during the breeding season (Packman Reference Packman2011). In this context, it is unsurprising that no Bengal Floricans were recorded in 2012 or 2018 at some of the smaller sites, nor that birds were lost from almost all of the small sites between 2012 and 2018. Of the four sites that still support Bengal Florican populations, only Baray District and Stoung-Chikraeng BFCA still contain more than 25 km2 grassland. Population estimates and trajectories in these two sites vary greatly (Figure 5), indicating that factors other than grass cover also influence population size. Non-breeding season habitat loss has been more severe in the area used by Bengal Floricans from Baray District than in the area used by the birds that breed in Stoung-Chikraeng BFCA and Sankor, which is protected as Trea Sameakki BFCA. Sub-optimal non-breeding habitat may lead to reduced breeding success in migratory birds (Norris et al. Reference Norris, Marra, Kyser, Sherry and Ratcliffe2004) and this may be true for Bengal Florican for which conditions in much of the area used during post-breeding dispersal are hostile (Newton Reference Newton2006).
The sex ratio recorded in Cambodia’s Bengal Florican population is a cause for concern because highly male-skewed sex ratios are typically associated with declining populations (Donald Reference Donald2007). In most adult bird species, males outnumber females by 2:1, although there may be a tendency towards female-biased sex ratios in lekking birds (Donald Reference Donald2007). Satellite telemetry indicates that female Bengal Floricans spend more time in the non-breeding grounds than males (Packman Reference Packman2011), which would make them more likely to be encountered during surveys of the non-breeding grounds. Unlike some other bustard species there is no evidence that in Bengal Florican the sexes differ in their choice of non-breeding habitat or location (Hillard Reference Hillard2012) in a way that might have affected detectability in our survey. Nonetheless, our estimate of the sex ratio in Cambodia’s Bengal Florican population should be considered provisional owing to the possibility that it is influenced by an unknown source of bias.
The apparent skewed sex ratio is probably caused by relatively high female mortality, because sex-biased survival is a strong predictor of adult sex ratios in birds (Székely et al. Reference Székely, Liker, Freckleton, Fichtel and Kappeler2014). There are a number of plausible causes of elevated female mortality in Bengal Floricans. Many local people work in or travel through the areas where Bengal Floricans breed, when people locate a ground-nesting bird’s nest the female is traditionally snared at the nest before the egg is taken (C. Hong pers. obs.). Although estimated annual survival rates are comparable to other bustards at 89.9% (82.2–97.6%; Mahood et al. Reference Mahood, Silva, Dolman and Burnside2016), small-scale local hunting may still occur at such a low rate that it is impossible to detect (Ibbett et al. Reference Ibbett, Lay, Phlai, Song, Hong, Mahood and Milner-Gulland2017). Free-ranging domestic dogs, which are a problem for many species (Doherty et al. Reference Doherty, Dickman, Glen, Newsome, Nimmo, Ritchie, Vanak and Wirsing2017) including bustards (Collar et al. Reference Collar, Patil and Bhardwaj2015), might also be significant predator of nests and incubating females; chewed feathers from a female Bengal Florican have been found at a failed nest site (S. Mahood and Son Virak pers. obs.). Conversely, mechanisation (which is positively associated with rural to urban migration) and a lack of access for cattle and their attendant herders may have incidentally reduced hunting levels at intensively farmed sites because there are now fewer people in the farmland.
With an estimated total population of just 138 (119–156) adults, the blandini subspecies of Bengal Florican is now the rarest bustard taxon globally and must now rank as one of the most at-risk bird taxa in the world. Below we detail site-specific threats and conservation activities for all of the remaining subpopulations.
The stable Bengal Florican population at Stoung-Chikraeng BFCA superficially represents the best chance for persistence of the subspecies. In contrast to other sites, Stoung-Chikraeng BFCA has been the focus for a joint Wildlife Conservation Society (WCS)–Ministry of Environment community-based conservation programme since 2005 (prior to 2016 the collaboration was with the Forestry Administration). Management of Stoung-Chikraeng BFCA is by a Community Management Committee, and activities include patrolling to prevent, detect and reverse grassland encroachment, a nest protection programme that pays community members for successful fledging of Bengal Florican nests, and an ecotourism scheme in which sightings of the Bengal Florican are linked to payments into a community fund that has been used to build toilets at the village school, repair the pagoda and purchase medicines. In the rice growing areas used by Bengal Floricans from Stoung-Chikraeng BFCA in the non-breeding season, a collaboration between WCS, local NGO Sansom Mlup Prey (SMP) and Mars Foods (a large-scale rice buyer) uses the Sustainable Rice Platform as a framework to test farming methods that create suitable habitat for Bengal Floricans and reduces chemical inputs, through land-levelling (which reduces the need for pesticides and herbicides) and carefully selected cover crops. Stoung-Chikraeng BFCA is evidence that it is possible for an NGO-government collaboration to stabilise trends in a population of a ‘Critically Endangered’ bird in a challenging context. The Bengal Florican is being promoted as a source of pride in Kampong Thom Province where Stoung-Chikraeng BFCA and the south-eastern populations are located, and an event celebrating the species was attended by more than 200 people in 2018. To mark the event, huge billboards depicting the species were erected alongside the main road, and photographs of Bengal Florican now hang in the office of the Kampong Thom Provincial Governor and in the office of the Ministry of Environment in Phnom Penh. The conservation programme at Stoung-Chikraeng BFCA costs approximately US$100,000 per year; financial constraints have prevented expanding this successful programme to Baray BFCA.
In the context of the successful conservation efforts described above, it is unfortunate that a high voltage power transmission line is currently under construction along the northern edge of the Tonle Sap floodplain, extremely close to Stoung-Chikraeng BFCA. All of Cambodia’s Bengal Florican will need to traverse the power line at least twice each year on their annual migration. Additionally, at Stoung-Chikraeng BFCA it is located close to an important lek and is predicted to significantly increase adult male mortality (Mahood et al. Reference Mahood, Silva, Dolman and Burnside2016). Even if technically feasible, financial considerations ruled out burying the power line to prevent bird collisions. Although Electricté du Cambodge have agreed to install bird flight deflectors on the stretch closest to Stoung-Chikraeng BFCA, collisions are still highly likely (Mahood et al. Reference Mahood, Silva, Dolman and Burnside2016). The construction of the power line could therefore end breeding at Stoung-Chikraeng BFCA and increase mortality in other populations. Given the potential for colonisation of newly created grassland, there is an urgent need to identify and rehabilitate abandoned or unproductive agricultural land near Stoung-Chikraeng BFCA to draw Bengal Floricans away from the area close to the power line.
The Bengal Florican population at Sankor is relatively large but declining rapidly. Sankor is unprotected, and already under intensive dry-season rice cultivation. In Sankor, WCS and SMP are investigating the appetite of AMRU (Cambodia’s largest rice exporter) and Mars Foods to mandate farmers to grow rice on rotation with fallows and a leguminous cover crop, an approach that would maintain soil quality and create habitat for Bengal Floricans. This potential intervention builds on the SRP pilot in the non-breeding grounds of the Bengal Floricans from Stoung-Chikraeng BFCA. Our results suggest that Bengal Floricans will move if habitat becomes unsuitable and fields are smaller than Bengal Florican display territories, therefore we theorise that the birds will be able to shift between fallow fields if they are created in a patchwork fashion. Moreover, other bustard species will breed successfully in young rotational fallows (Morales et al. Reference Morales, Traba, Delgado and Morena2013), and legumes have previously been used as a tool to reverse bustard declines (Bretagnolle et al. Reference Bretagnolle, Villers, Denonfoux, Cornulier, Inchausti and Badenhausser2011). If these proposed measures are not acceptable to farmers or rice buyers, they fail to create suitable habitat for Bengal Floricans or if they are implemented slowly, then the species is likely to abandon Sankor within a few years.
The Bengal Florican population in Baray District is located mostly to the south-east of the BFCA, in an area that is currently too distant from irrigation infrastructure to be converted to dry-season rice. However, there are plans to construct irrigation infrastructure to the east of Baray BFCA in 2019–2021, which, if not very carefully managed and potential impacts mitigated, may lead to the extinction of Bengal Florican in this site. In this context, sensitive development is critical, and there is an opportunity to promote a combination of protected area management similar to that employed at Stoung-Chikraeng BFCA, and private-sector led farming following the principles of the Sustainable Rice Platform, to give this Bengal Florican population a chance to thrive.
In Bakan WCS are working with the Ministry of Environment to establish a protected area. At the same time, SMP, a local NGO, are promoting cultivation of deep-water rice and developing a niche agricultural product that will provide financial incentives to farmers to grow this low GI rice strain. Even if these actions are too late for this tiny population of Bengal Florican, Bakan is the best remaining example of inner floodplain grassland in the Tonle Sap floodplain and supports a suite of species that make it worth protecting regardless of the presence of Bengal Florican. Compared to other sites in the Tonle Sap floodplain, Bakan still supports large numbers of grassland passerines, including the largest wintering population of ‘Critically Endangered’ Yellow-breasted Bunting Emberiza aureola in Cambodia, and is one of only two known locations with a population of Chinese Grassbird Graminicola striatus in South-East Asia (Eaton et al. Reference Eaton, Mahood and Eames2014).
We cannot rule out the possibility that there is a small number of Bengal Floricans in agricultural areas that we did not survey, but there is no reason to suggest that these undiscovered birds are even close to equal in number to those that are unaccounted for since 2012. Although it is plausible that Bengal Floricans disperse when grassland is destroyed, ongoing analysis of satellite images indicates that there are no large unsurveyed patches of grassland in the Tonle Sap floodplain that they could colonise. The only recent record of H. b. blandini outside the Tonle Sap floodplain was a female with chick in August 2015 at Bueng Prek Lapouv close to the international border with Vietnam (J. C. Eames pers. comm.). This was the first record in the Mekong Delta for a number of years, despite frequent visits to Bueng Prek Lapouv and the nearby Anlung Pring Sarus Crane Conservation Area since the mid-2000s as part of an ongoing conservation project by BirdLife International and WWT. In the mid-2000s the population at Bueng Prek Lapouv was estimated at 2–3 displaying males (Gray et al. Reference Gray, Collar, Davidson, Dolman, Evans, Fox, Chamnan, Borey, Hout and Van Zalinge2009b). There appear to have been no records of Bengal Florican from Vietnam since the late 1990s (Donald et al. Reference Donald, Collar, Marsden and Pain2013). Although the recent Bueng Prek Lapouv record suggests that small numbers of Bengal Floricans can persist unnoticed for many years, the Mekong Delta population is evidently likely almost extirpated.
Given the small size of Cambodia’s Bengal Florican population and the situation at the sites outlined above, captive breeding has been suggested as a solution. However, we caution that demographic modelling, or at least a thorough evaluation of potential impacts on wild populations and possible outcomes of captive breeding, is a prerequisite before embarking on a long and expensive programme of ex-situ conservation for a species that has never been kept in captivity (Dolman et al. Reference Dolman, Collar, Scotland and Burnside2015). Although desirable, it has not been possible to increase the size of existing BFCAs, designate additional BFCAs or purchase agricultural land and convert it to grassland (Packman et al. Reference Packman, Showler, Collar, Virak, Mahood, Handschuh, Evans, Chamnan and Dolman2013b). Land prices in the Tonle Sap floodplain are very high so millions of dollars would be needed to purchase enough for a small grassland reserve. Furthermore, land tenure is not secure so understandably investors cannot be found. Also converting agricultural land that produces food for local consumption or export to unproductive grassland goes against Cambodia’s Rectangular Strategy, which guides sectoral policy and prioritises economic growth (Royal Government of Cambodia 2013). Bengal Florican conservation must be implemented in the context of the development and management of the Tonle Sap floodplain, within which more than 2 million people live. The rapidly changing economic needs of these people, as well as the predicted impacts of climate change and hydropower dams on vegetation and farming (Arias et al. Reference Arias, Cochrane, Piman, Kummu, Caruso and Killeen2012, Reference Arias, Piman, Lauri, Cochrane and Kummu2014), must be taken into account when designing and implementing conservation measures. In this context the only hope for Bengal Florican populations outside of the BFCAs lies in working with the private sector to incentivize farmers to grow rice in a way that enables Bengal Florican to persist. Given the area requirements of Bengal Florican, this must be done at scale, and quickly.
The population of Bengal Florican in India is estimated at 147–198 adult territorial males (or 350–400 individuals assuming an equal sex ratio) and the total population in Nepal is thought to be fewer than 100 individuals (Collar et al. Reference Collar, Baral, Batbayer, Bhardwaj, Brahma, Burnside, Choudhury, Combreau, Dolman, Donald, Dutta, Gadhavi, Gore, Goroshko, Hong, Jather, Jha, Jhala, Koshkin, Lahkar, Liu, Mahood, Morales, Narwade, Natsagdorj, Nefedov, Silva, Thakuri, Wang, Zhang and Kessler2017). In Cambodia, although there is significant inter-annual variation in population estimates within sites (Figure 4), population size and trends of Bengal Florican are well understood owing to intensive long-term monitoring using a standardised methodology that minimises the potential for double-counting of males. In contrast, populations in India and Nepal are monitored less frequently and utilise a number of different methods; for instance, in Nepal some sites are surveyed using “sweep counts” that aim to flush birds in a similar way to the non-breeding season census we conducted in Cambodia, whilst at other sites and in India displaying males are located and territories mapped accordingly (Rahmani et al. Reference Rahmani, Khongsai, Rahman, Imran, Sagwan and Ojah2016a, Reference Rahmani, Rahman, Imran, Sagwan and Khongsai2016b, Jha et al. Reference Jha, Thakuri, Rahmani, Dhakal, Khongsai, Pradhan, Shinde, Chauhan, Talegaonkar and Barber2018). Although in some circumstances these methods are more likely to give accurate population estimates, they are also more likely to double-count males and, because they are not standardised, trends are harder to assess. It is possible that populations in some sites in India and Nepal may be overestimated and declines might be obscured until they are severe. Safety considerations specifically related to the presence of Tiger Panthera tigris, Asian Elephant Elephas maximus and Indian rhinoceros Rhinoceros unicornis, preclude monitoring on foot following exactly the same method used in Cambodia. Habitats also differ from Cambodia; the sites where Bengal Florican occurs in India and Nepal are a heterogeneous mix consisting of large areas of floodplain grassland within a matrix of deciduous forest (Rahmani et al. Reference Rahmani, Khongsai, Rahman, Imran, Sagwan and Ojah2016a). Given the declines experienced by all other South and South-East Asian bustard taxa, we recommend testing a modification of the Cambodian method, in which grasslands are mapped, 1 km2 survey squares randomly allocated across grassland and surveyed as in Cambodia (but from observation towers, the roofs of vehicles and elephant-back rather than on foot) and these data used to calculate population estimates (displaying males) as in Cambodia but using area of grassland rather than area of site. In addition, because population estimates for H. b. bengalensis assume an equal sex ratio, the actual population may be considerably lower if sex ratios are similar to those recorded in Cambodia. There is an urgent need to evaluate the sex ratio of Bengal Florican populations in India and Nepal.
The current total population, contraction of range and rate of decline of H. b. blandini are similar to, and as concerning as, those experienced by the Great Indian Bustard Ardeotis nigriceps and Lesser Florican Sypheotides indicus. Like Cambodia’s Bengal Florican population, the Great Indian Bustard declined from a few thousand in the 1980s and 1990s to 600–700 by around 2000; by 2010 the population was thought to be just 300 individuals spread across eight sites (Dutta et al. Reference Dutta, Rahmani and Jhala2010). By 2014 the population had declined to 200 individuals (Collar et al. Reference Collar, Patil and Bhardwaj2015), and in 2017 it was estimated that only 170 ± 63 adults remained in just a fraction of its former range, with most of the population at just one site (Collar et al. Reference Collar, Baral, Batbayer, Bhardwaj, Brahma, Burnside, Choudhury, Combreau, Dolman, Donald, Dutta, Gadhavi, Gore, Goroshko, Hong, Jather, Jha, Jhala, Koshkin, Lahkar, Liu, Mahood, Morales, Narwade, Natsagdorj, Nefedov, Silva, Thakuri, Wang, Zhang and Kessler2017). Lesser Florican also underwent a similar, but less well studied, decline during the same period, dropping from 1,103–1,765 males during 1994–1999 to about 270 males in 2017 (Dutta et al. Reference Dutta, Narwade, Bipin, Gadhavi, Uddin, Mhasker, Pandey, Mohan, Sharma, Iyer, Tripathi, Verma, Varma, Chakdar, Karulkar, Khongsai, Kumar, Gore, Jhala, Vaidya, Horne, Chittora, Annigeri, Trivedi and Jhala2018). All three species have been massively impacted by loss of grassland and intensification of agriculture (Collar et al. Reference Collar, Baral, Batbayer, Bhardwaj, Brahma, Burnside, Choudhury, Combreau, Dolman, Donald, Dutta, Gadhavi, Gore, Goroshko, Hong, Jather, Jha, Jhala, Koshkin, Lahkar, Liu, Mahood, Morales, Narwade, Natsagdorj, Nefedov, Silva, Thakuri, Wang, Zhang and Kessler2017).
The conservation measures described here represent the last hope for the blandini subspecies of Bengal Florican and will have to be pursued vigorously to prevent its extinction within five years. The causes of its catastrophic decline are extremely similar to those that imperil Great Indian Bustard, although collisions with power lines have played a more important role in the decline of that species (Collar et al. Reference Collar, Patil and Bhardwaj2015), at least until now (Mahood et al. Reference Mahood, Silva, Dolman and Burnside2016). There may also be similarities to the factors threatening the poorly known Lesser Florican, which is also largely restricted to farmland outside of protected areas. Bengal Floricans in India and Nepal breed almost entirely in protected areas but spend the non-breeding season in farmland, so they may face similar threats (Jha et al. Reference Jha, Thakuri, Rahmani, Dhakal, Khongsai, Pradhan, Shinde, Chauhan, Talegaonkar and Barber2018). Successful community-based conservation models implemented at Stoung-Chikraeng BFCA could possibly be modified and applied in India and Nepal to reduce the chance of extinction of those bustard taxa. Collaboration between bustard conservationists in Asia is essential to refine monitoring methods, reduce duplication of research and rapidly apply conservation lessons. Whilst losing just one of these bustard taxa would be a tragedy, failing to work together increases the risk of extinction for all. Preventing the extinction of these charismatic bustards is one of the greatest priorities, and biggest challenges, facing conservation in Asia.
Acknowledgements
We are very grateful to Hun Sophea, Noun Chandany and Heam Phanny for assistance with field data collection. Kong Sophalrachana helped with the map. We thank the Department of Freshwater Wetlands Conservation in the General Department of Administration for Nature Conservation and Protection, Ministry of Environment, and the Siem Reap and Kampong Thom Provincial Governments for their support. This paper benefitted greatly from the comments of two anonymous reviewers. The 2018 Bengal Florican survey was supported by grant no. 65893 from the Critical Ecosystem Partnership Fund (a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the John D. and Catherine T. MacArthur Foundation and the World Bank; a fundamental goal is to ensure civil society is engaged in biodiversity conservation). S. P. M. is the recipient of a Prestigious International Research Tuition Scholarship at Charles Darwin University.












