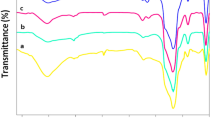
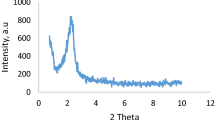
Abstract
In this study, ordered structure with a hexagonal arrangement MCM-41 was synthesized and functionalized with L-cysteine group and further palladium particles has been supported on its surface (L-cysteine-Pd@MCM-41). The prepared catalyst is well characterized by various techniques such as low-angle X-ray diffraction (XRD), thermal gravimetric analysis (TGA), Fourier transform infrared spectroscopy (FT-IR), N2 adsorption–desorption measurement isotherms, energy-dispersive X-ray spectroscopy (EDS), X-ray Mapping (WDX), scanning electron microscopy (SEM) and inductively coupled plasma (ICP) techniques. The collected data from all techniques provided evidence that palladium complex was immobilized onto MCM-41 pores. The obtained nanocatalyst demonstrated excellent activity as a new heterogeneous catalyst for the homoselective synthesis of 5-substituted 1H-tetrazole derivatives. This method has the advantages of simple methodology, eco-friendly and simplicity in the separation of catalyst, shorter reaction times, and high yields of the product. Finally, easy catalyst recovery was achieved and the catalyst could be recycled for six times without a significant decrease in activity and selectivity.













Similar content being viewed by others
References
H. Hamidi, M.M. Heravi, M. Tajbakhsh, M. Shiri, H.A. Oskooie, S.A. Shintre, N.A. Koorbanally, J. Iran Chem. Soc. 12, 2205 (2015)
A.R. Moosavi-Zare, H. Goudarziafshar, Z. Jalilian, Prog. Chem. Biochem. Res. 2, 59 (2019)
A. Ghorbani-Choghamarani, M.A. Zolfigol, M. Hajjami, H. Goudarziafshar, M. Nikoorazm, S. Yousefi, B. Tahmasbi, J. Braz. Chem. Soc. 22, 525 (2011)
S. Taghavi Fardood, A. Ramazani, F. Moradnia, Z. Afshari, S. Ganjkhanlu, F. Yekke Zare, Green Synthesis of ZnO Nanoparticles via Sol-gel Method and Investigation of Its Application in Solvent-free Synthesis of 12-Aryl-tetrahydrobenzo [α] xanthene-11-one Derivatives Under Microwave Irradiation. Chem. Methodol. 3(6), 696–706 (2019)
B. Atashkar, A. Rostami, H. Gholami, B. Tahmasbi, Res. Chem. Intermed. 41, 3675 (2015)
M. Shiri, M.M. Heravi, H. Hamidi, M.A. Zoligol, Z. Tanbakouchian, A. Nejatinezhad-Arani, S.A. Shintre, N.A. Koorbanally, Transition metal-free synthesis of quinolino [2′, 3′: 3, 4] pyrazolo [5, 1-b] quinazolin-8 (6H)-ones via cascade dehydrogenation and intramolecular N-arylation. J. Iran Chem. Soc. 13(12), 2239–2246 (2016)
A. Ramazani, M. Khoobi, A. Torkaman, F. Zeinali Nasrabadi, H. Forootanfar, M. Shakibaie, M. Jafari, A. Ameri, S. Emami, M.A. Faramarzi, A. Foroumadi, A. Shafiee, One-pot, four-component synthesis of novel cytotoxic agents 1-(5-aryl-1, 3, 4-oxadiazol-2-yl)-1-(1H-pyrrol-2-yl) methanamines. Eur. J. Med. Chem. 78, 151–156 (2014)
A. Ghorbani-Choghamarani, P. Moradi, B. Tahmasbi, Polyhedron 163, 98 (2019)
H. Aghahosseini, A. Ramazani, N. Safarvand Jalayer, Z. Ranjdoost, A. Souldozi, K. Slepokura, T. Lis, Org. Lett. 21, 22 (2019)
A. Sarvary, A. Maleki, Mol. Divers. 19, 189 (2015)
A. Teimouri, A. Najafi Chermahini, Polyhedron 30, 2606 (2011)
A. Maleki, R. Taheri-Ledari, R. Ghalavand, R. Firouzi-Haji, J. Phys. Chem. Solids 136, 109200 (2020)
A. Maleki, A. Sarvary, RSC Adv. 5, 60938 (2015)
F. Ek, L.G. Wistrand, T. Frejd, Tetrahedron 59, 6759 (2003)
B.S. Jursic, B.W.J. LeBlanc, Heterocyclic. Chem. 35, 405 (1998)
M. Nasrollahzadeh, Y. Bayat, D. Habibi, S. Moshaee, Tetrahedron. Lett. 50, 4435 (2009)
R. Shelkar, A. Singh, J. Nagarkar, Tetrahedron. Lett. 54, 106 (2013)
G. Venkateshwarlu, A. Premalatha, K.C. Rajanna, P.K. Saiprakash, Synthetic. Commun. 39, 4479 (2009)
F. Dehghani, A.R. Sardarian, M. Esmaeilpour, J. Organomet. Chem. 743, 87 (2013)
M. Abdollahi-Alibeik, A. Moaddeli, New. J. Chem. 39, 2116 (2015)
A. Hassankhani, B. Gholipour, S. Rostamnia, Polyhedron 175, 114217 (2020)
S.M. Agawane, J.M. Nagarkar, Catal. Sci. Technol. 2, 1324 (2012)
A. Kumar, R. Narayanan, H. Shechter, J. Org. Chem. 61, 4462 (1996)
D. Habibi, M. Nasrollahzadeh, Y. Bayat, Synthetic. Commun. 41, 2135 (2011)
R.M. Herbst, K.R. Wilson, J. Org. Chem. 22, 1142 (1957)
D. Amantini, R. Beleggia, F. Fringuelli, F. Pizzo, L. Vaccaro, J. Org. Chem. 69, 2896 (2004)
M. Lakshmi Kantam, K.B. Shiva Kumar, C. Sridhar, Adv. Synth. Catal. 347(9), 1212–1214 (2005)
T. Jin, F. Kitahara, S. Kamijo, Y. Yamamoto, Tetrahedron. Lett. 49, 2824 (2008)
M.L. Kantam, K. B. Shiva Kumar, K. Phani Raja (2006) J. Mol. Catal. A. Chem. 247(1): 186–188
B. Tahmasbi, A. Ghorbani-Choghamarani, New J. Chem. 43, 14485 (2019)
V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara, J.M. Basset, Chem. Rev. 111, 3036 (2011)
A.N. Chermahini, A. Teimouri, A. Moaddeli, Heteroatm. Chem. 22, 168 (2011)
D.E. De Vos, M. Dams, B.F. Sels, P.A. Jacobs, Chem. Rev. 102, 3615 (2002)
J. Evans, A.B. Zaki, M.Y. El-Sheikh, S.A. El-Safty, J. Phys. Chem. B. 104, 10271 (2000)
A. Ahadi, H. Alamgholiloo, S. Rostamnia, X. Liu, M. Shokouhimehr, D.A. Alonso, R. Luque, ChemCatChem 11, 4803 (2019)
E. Doustkhah, J. Lin, S. Rostamnia, C. Len, R. Luque, X. Luo, Y. Bando, K.C.-W. Wu, J. Kim, Y. Yamauchi, Y. Ide, Chem. Eur. J. 25, 1614 (2019)
H. Golchin Hosseini, E. Doustkhah, M.V. Kirillova, S. Rostamnia, G. Mahmoudi, A.M. Kirillov, Appl. Catal. A 548, 96–97 (2017)
E Doustkhah, S. Rostamnia, H. Golchin Hosseini, R. Luque (2017) Chemistryselect 2(1): 329–334
E. Doustkhah, S. Rostamnia, A. Hassankhani, J. Porous Mater. 23, 549 (2016)
K. Dhara, K. Sarkar, D. Srimani, S. Kumar Saha, P. Chattopadhyay, A. Bhaumik, Dalton. Trans. 39(28), 6395 (2010)
S. Jana, S. Bhunia, B. Dutta, S. Koner, Appl. Catal. A: Gen. 392, 225 (2011)
E. Doustkhah, H. Mohtasham, M. Farajzadeh, S. Rostamnia, Y. Wang, H. Arandiyan, M.H.N. Assadi, Micropor Mesopor Mat. 293, 109832 (2020)
S. Rostamnia, E. Doustkhah, H. Golchin Hosseini, H. Behzad Zeynizadeh, R.L. Xin, Catal. Sci. Technol. 6(1), 4124 (2016)
S. Rostamnia, Turaj Rahmani. Appl. Organometal. Chem. 29, 471 (2015)
A.R. Abbasi, H. Kalantary, M. Yousefi, A. Ramazani, A. Morsali, Ultrason. Sonochem. 19, 853 (2012)
M. Nikoorazm, A. Ghorbani-Choghamaranai, M. Khanmoradi, P. Moradi, J. Porous Mater. 25, 1831 (2018)
M. Nikoorazm, A. Ghorbani-Choghamarani, A. Panahi, B. Tahmasbi, N. Noori, J. Iran. Chem. Soc. 15, 181 (2018)
M. Nikoorazm, M. Khanmoradi, J. Chin. Chem. Soc. (2020). https://doi.org/10.1002/jccs.201900531
A. Ghorbani-Choghamarani, M. Hajjami, B. Tahmasbi, N. Noori, J. Iran. Chem. Soc. 13, 2193 (2019)
P. Moradi, M. Hajjami, F. Valizadeh-Kakhki, Appl. Organometal. Chem. 33, e5205 (2019)
M. Nikoorazm, A. Ghorbani-Choghamarani, N. Noori, B. Tahmasbi, Appl. Organometal. Chem. 30, 843 (2016)
A. Ghorbani-Choghamarani, P. Moradi, B. Tahmasbi, J. Iran. Chem. Soc. 16, 511 (2019)
H. Xie, H. Liu, M. Wang, H. Pan, C. Gao, Appl. Organometal. Chem. 34, e5256 (2020)
M. Nikoorazm, N. Noori, S. Faryadi, B. Tahmasbi, Transit Met Chem. 42, 469 (2017)
P. Moradi, M. Hajjami, B. Tahmasbi, Polyhedron 175, 114169 (2020)
R. Shaikh, I. Zainuddin Syed, P. Bhende, Asian J. Green Chem. 3(1), 70 (2019)
H. Alamgholiloo, S. Rostamnia, A. Hassankhani, X. Liu, A. Eftekhari, A. Hasanzadeh, K. Zhang, H. Karimi-Maleh, S. Khaksar, R.S. Varma, M. Shokouhimehr, J. Colloid Interface Sci. 567, 126 (2020)
P. Zhao, H. Yin, H. Gao, C. Xi, J. Org. Chem. 78, 5001 (2013)
A. Ghorbani-Choghamarani, L. Shiri, G. Azadi, Res. Chem. Intermed. 42, 6049 (2016)
A. Ghorbani-Choghamarani, G. Azadi, Appl. Organometal. Chem. 30, 247 (2016)
H.H. Thacker, V.R. Ram, P.N. Dave, Prog. Chem. Biochem. Res. 2(1), 84 (2019)
S. Taghavi Fardood, A. Ramazani, S. Moradi, P. Azimzadeh Asiabi (2017) J. Mater. Sci: Mater. Electron. 28(3): 13596
M. Nikoorazm, M. Khanmoradi, M. Mohammadi, Appl. Organometal. Chem. 34, e5504 (2020)
A. Ghorbani-Choghamarani, B. Tahmasbi, R.H.E. Hudson, A. Heidari, Micropor Mesopor Mat. 284, 366 (2019)
N. Razavi, B. Akhlaghinia, RSC. Adv. 5(35), 87769 (2015)
A. Khalafi-Nezhad, S. Mohammadi, RSC. Adv. 3, 4362 (2013)
P. Sivaguru, P. Theerthagiri, A. Lalitha, Tetrahedron. Lett. 56, 2203 (2015)
M. Nikoorazm, Z. Rezaei, B. Tahmasbi, J Porous Mater (2020). https://doi.org/10.1007/s10934-019-00835-6
F. Abrishami, M. Ebrahimikia, F. Rafiee, Appl. Organomet. Chem. 29, 730 (2015)
B. Tahmasbi, A. Ghorbani-Choghamarani, P. Moradi, New. J. Chem 44, 3717 (2020)
M. Hosseini-Sarvari, S. Najafvand-Derikvandi, C. R. Chimie. 17, 1007 (2014)
A. Khalafi-Nezhad, S. Mohammadi, RSC. Adv 3, 4362 (2013)
P. Sivaguru, P. Theerthagiri, A. Lalitha, Tetrahedron. Lett 56, 2203 (2015)
ML Kantam, K.B. Shiva Kumar, K.J. Phani Raja (2006) J. Mol. Catal. A: Chem. 247(2): 186.
M. Abdollahi-Alibeik, A. Moaddeli, New. J. Chem 39, 2116 (2015)
A. Ghorbani-Choghamarani, P. Moradi, B, Tahmasbi. RSC. Adv 6, 56638 (2016)
B. Tahmasbi, A. Ghorbani-Choghamarani, Appl. Organometal. Chem 31, e3644 (2017)
P. Moradi, A. Ghorbani-Choghamarani, Appl. Organometal. Chem 31, e3602 (2017)
D. Habibi, M. Nasrollahzadeh, L. Mehrabi, S. Mostafaee, Monatsh Chem 144, 725 (2013)
E. Erken, İ. Esirden, M. Kaya, F. Sen, RSC Adv. 5, 68558 (2015)
Acknowledgements
This work was supported by the research facilities of Ilam University, Ilam, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nikoorazm, M., Moradi, P. & Noori, N. L-cysteine complex of palladium onto mesoporous channels of MCM-41 as reusable, homoselective and organic–inorganic hybrid nanocatalyst for the synthesis of tetrazoles. J Porous Mater 27, 1159–1169 (2020). https://doi.org/10.1007/s10934-020-00894-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-020-00894-0