Abstract
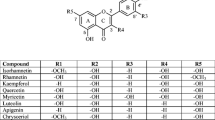
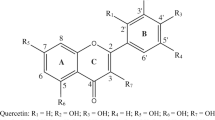
Quercetin is the most abundant flavonoid with potent antioxidant activities. In the current research, the antioxidant properties of quercetin and quercetin-DNA complex were investigated theoretically and experimentally. Free radical scavenging experiments with thiobarbituric acid–reactive substances (TBARS) and 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH) indicate that quercetin can protect DNA from free radical damage, and the antioxidant activity of the quercetin-DNA complex is stronger than quercetin. Deoxyriboseadenine-quercetin-dimethylphosphinic acid (DA-Q-P) model was extracted from molecular docking. The contributions of hydroxyl groups in quercetin and DA-Q-P model molecules to the antioxidant activity were investigated by computation of bond dissociation enthalpy (BDE) parameter and Fukui function, at B3LYP/6-311++G(2d,2p) level of theory. The results outlined that the hydroxyl groups from the B ring (3′-OH and 4′-OH) have a lower BDE compared with the ones from the A and C rings (3-OH, 5-OH, and 7-OH) and hence define antioxidant activity. The computational result based on Fukui function shows that the B ring is an electrophilic region. The interaction of antioxidant with DNA discovered at the molecular level could provide the structural basis of the antioxidant property of active ingredients in the flavonoids. It is of great significance to study the interaction mechanism between the small drug molecules with DNA at the molecular level.






Similar content being viewed by others
References
Valacchi G, Davis P (2008) Oxidants in biology: a question of balance. Springer, Dordrecht
Boots AW, Haenen GR, Bast A (2008) Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 585(2–3):325–337
Kim GN, Jang HD (2009) Protective mechanism of quercetin and rutin using glutathione metabolism on H2O2-induced oxidative stress in HepG2 cells. Ann N Y Acad Sci 1171(1):530–537
Robaszkiewicz A, Balcerczyk A, Bartosz G (2007) Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol Int 31(10):1245–1250
Xu D, Hu M-J, Wang Y-Q, Cui Y-L (2019) Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 24(6):1123
Selvaraju V, Joshi M, Suresh S, Sanchez JA, Maulik N, Maulik G (2012) Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease–an overview. Toxicol Mech Methods 22(5):330–335
Stone MP, Huang H, Brown KL, Shanmugam G (2011) Chemistry and structural biology of DNA damage and biological consequences. Chem Biodivers 8(9):1571–1615
Martinez A, Kolter R (1997) Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J Bacteriol 179(16):5188–5194
Wu Z, Zhen Z, Jiang J-H, Shen G-L, Yu R-Q (2009) Terminal protection of small-molecule-linked DNA for sensitive electrochemical detection of protein binding via selective carbon nanotube assembly. J Am Chem Soc 131(34):12325–12332
Hori Y, Otomura N, Nishida A, Nishiura M, Umeno M, Suetake I, Kikuchi K (2018) Synthetic-molecule/protein hybrid probe with fluorogenic switch for live-cell imaging of DNA methylation. J Am Chem Soc 140(5):1686–1690
Kumar C, Asuncion EH (1993) DNA binding studies and site selective fluorescence sensitization of an anthryl probe. J Am Chem Soc 115(19):8547–8553
Dervan PB (2001) Molecular recognition of DNA by small molecules. Bioorg Med Chem 9(9):2215–2235
Kanakis C, Tarantilis P, Polissiou M, Diamantoglou S, Tajmir-Riahi H (2005) DNA interaction with naturally occurring antioxidant flavonoids quercetin, kaempferol, and delphinidin. J Biomol Struct Dyn 22(6):719–724
Kanakis CD, Tarantilis PA, Polissiou M, Diamantoglou S, Tajmir-Riahi H (2007) An overview of DNA and RNA bindings to antioxidant flavonoids. Cell Biochem Biophys 49(1):29–36
Das A, Majumder D, Saha C (2017) Correlation of binding efficacies of DNA to flavonoids and their induced cellular damage. J Photochem Photobiol B Biol 170:256–262
Sha Y, Chen X, Niu B, Chen Q (2017) The interaction mode of groove binding between quercetin and calf thymus DNA based on spectrometry and simulation. Chem Biodivers 14(10):e1700133
Srivastava S, Somasagara RR, Hegde M, Nishana M, Tadi SK, Srivastava M, Choudhary B, Raghavan SC (2016) Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep 6:24049
Vitorino J, Sottomayor M (2010) DNA interaction with flavone and hydroxyflavones. J Mol Struct 975(1–3):292–297
Liu Y, Wu X, Zhou H, Liu X, Zhang F, Yang J (2009) The fluorescence enhancement of quercetin–nucleic acid system and the analytical application. Lumin J Biol Chem Lumin 24(6):416–421
Bi S, Qiao C, Song D, Tian Y, Gao D, Sun Y, Zhang H (2006) Study of interactions of flavonoids with DNA using acridine orange as a fluorescence probe. Sensors Actuators B Chem 119(1):199–208
Lu X, Chen Y, Chen J, Zhang Y, Zhang L, Li M (2006) Electrochemical studies of the interaction of quercetin with DNA. Int J Electrochem Sci 1:130–138
Zhu Z, Li C, Li N-Q (2002) Electrochemical studies of quercetin interacting with DNA. Microchem J 71(1):57–63
Oliveira-Brett AM, Diculescu VC (2004) Electrochemical study of quercetin–DNA interactions. Part I. Analysis in incubated solutions. Bioelectrochemistry 64(2):133–141
Silva JP, Gomes AC, Coutinho OP (2008) Oxidative DNA damage protection and repair by polyphenolic compounds in PC12 cells. Eur J Pharmacol 601(1–3):50–60
Janjua NK, Siddiqa A, Yaqub A, Sabahat S, Qureshi R, ul Haque S (2009) Spectrophotometric analysis of flavonoid–DNA binding interactions at physiological conditions. Spectrochim Acta A Mol Biomol Spectrosc 74(5):1135–1137
Cao W, Chen W, Zheng X, Zheng J (1956) Modified method to evaluate the protection of the antioxidants against hydroxyl radical-mediated DNA damage. Acta Nutri Sin 01
Fukumoto L, Mazza G (2000) Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48(8):3597–3604
Zhou F, Wang J, Zhang Y, Wang Q, Guo C, Wang F, Zheng X, Zhang H (2019) Theoretical studies on the bond strength and electron density characteristics in multiple hydrogen bonded arrays. J Mol Graph Model 107439
Bardak F (2019) Experimental and DFT analysis of structural and spectroscopic features of nitroterephthalic acid, and computational insights into its molecular interactions with hER-α via molecular docking. J Mol Struct 1175:458–470
Abraham CS, Muthu S, Prasana JC, Armaković S, Armaković SJ, Geoffrey B (2019) Computational evaluation of the reactivity and pharmaceutical potential of an organic amine: a DFT, molecular dynamics simulations and molecular docking approach. Spectrochim Acta A Mol Biomol Spectrosc 222:117188
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19(14):1639–1662
Frisch MJ, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2009) Gaussian 09, Revision D. 01. Gaussian. Inc., Wallingford
Becke AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98(2):1372–1377
Stephens PJ, Devlin F, Chabalowski C, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98(45):11623–11627
Miertuš S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem Phys 55(1):117–129
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Foti MC, Daquino C (2011) Kinetics of the oxidation of quercetin by 1,1-diphenyl-2-picrylhydrazyl (dpph•)
Sak K (2014) Dependence of DPPH radical scavenging activity of dietary flavonoid quercetin on reaction environment. Mini-Rev Med Chem 14(6):494–504
Maciel EN, Almeida SK, da Silva SC, de Souza GL (2018) Examining the reaction between antioxidant compounds and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) through a computational investigation. J Mol Model 24(8):218
Mertens-Talcott SU, Percival SS (2005) Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett 218(2):141–151
Yang F, Song L, Wang H, Wang J, Xu Z, Xing N (2015) Combination of quercetin and 2-methoxyestradiol enhances inhibition of human prostate cancer LNCaP and PC-3 cells xenograft tumor growth. PLoS One 10(5):e0128277
Michalík M, Rimarčík J, Lukeš V, Klein E (2019) Thermodynamics of primary antioxidant action of flavonols in polar solvents. Acta Chim Slov 12(1):108–118
Rizwana BF, Muthu S, Prasana JC, Abraham CS, Raja M (2018) Spectroscopic (FT-IR, FT-Raman) investigation, topology (ESP, ELF, LOL) analyses, charge transfer excitation and molecular docking (dengue, HCV) studies on ribavirin. Chem Data Collect 17-18:236–250
Ramesh P, Lydia Caroline M, Muthu S, Narayana B, Raja M, Aayisha S (2020) Spectroscopic and DFT studies, structural determination, chemical properties and molecular docking of 1-(3-bromo-2-thienyl)-3-[4-(dimethylamino)-phenyl]prop-2-en-1-one. J Mol Struct 1200:127123
B FR, Prasana JC, Muthu S, Abraham CS (2019) Molecular docking studies, charge transfer excitation and wave function analyses (ESP, ELF, LOL) on valacyclovir : a potential antiviral drug. Comput Biol Chem 78:9–17
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, X., Wang, Y. & Gao, L. Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. J Mol Model 26, 133 (2020). https://doi.org/10.1007/s00894-020-04356-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04356-x