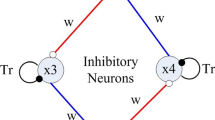
Using a simulation approach, we propose a biophysically feasible mechanism describing how a multifunctional central pattern generator (CPG) can produce co-existing slow and fast rhythms of the patterned motor output. This mechanism suggests that the same core elemental CPG can produce either a locomotion or a paw shaking rhythm in mammals (cat). We built a Hodgkin–Huxley-style biophysical model that generates multistability of a locomotion-like regime and a paw shake-like regime. This model is constructed as a half-center oscillator (HCO), in which two inhibitory neurons (representing the respective neuronal populations in real CPGs) reciprocally inhibit each other. We propose that the locomotion rhythm and the paw shaking rhythm are controlled by two different slowly inactivating intrinsic ion currents. In our model, slowly inactivating low voltage-activated calcium current (ICaS) drives the locomotion-like regime, while slowly inactivating sodium current (INaS) drives the paw shakelike regime. We investigated whether asymmetric characteristics of these regimes could be reliably and separately controlled by asymmetric variation of the conductances of these two currents. We found that variation of the conductance of ICaS in only one neuron, while holding this conductance constant in the other neuron, produces asymmetric changes in bursting characteristics, including the burst duration and interburst interval of the locomotion-like regime without producing notable changes of the paw shake-like regime. We also found that similar variation of the conductance of INaS affects the bursting characteristics of both regimes, although the paw shake-like regime is affected more remarkably than the locomotion-like one.
Similar content being viewed by others
References
S. Yakovenko, D. A. McCrea, K. Stecina, et al., “Control of locomotor cycle durations,” J. Neurophysiol., 94, No. 2, 1057–1065 (2005).
A. Frigon and J. P. Gossard, “Asymmetric control of cycle period by the spinal locomotor rhythm generator in the adult cat,” J. Physiol., 587, Pt. 19, 4617–4628 (2009).
B. Bondy, A. N. Klishko, D. H. Edwards, et al., “Control of cat walking and paw-shake by a multifunctional central pattern generator,” in: Neuromechanical Modeling of Posture and Locomotion, B. I. Prilutsky and D. H. Edwards (eds.), Springer, New York (2016), pp. 333–359.
J. Parker, B. Bondy, B. I. Prilutsky, et al., “Control of transitions between locomotor-like and paw shakelike rhythms in a model of a multistable central pattern generator,” J. Neurophysiol., 120, No. 3, 1074–1089 (2018).
R. L. Calabrese, F. Nadim, and O. H. Olsen, “Heartbeat control in the medicinal leech: a model system for understanding the origin, coordination, and modulation of rhythmic motor patterns,” J. Neurobiol., 27, No. 3, 390–402 (1995).
G. S. Cymbalyuk, Q. Gaudry, M. A. Masino, et al., “Burst-ing in leech heart interneurons: cell-autonomous and network-based mechanisms,” J. Neurosci., 22, No. 24, 10580–10592 (2002).
A. A. Hill, J. Lu, M. A. Masino, et al., “A model of a segmental oscillator in the leech heartbeat neuronal network,” J. Comput. Neurosci., 10, No. 3, 281–302 (2001).
D. A. McCrea and I. A. Rybak, “Modeling the mammalian locomotor CPG: insights from mistakes and perturbations,” Prog. Brain Res., 165, 235–253 (2007).
I. A. Rybak, N. A. Shevtsova, M. Lafreniere-Roula, et al., “Modelling spinal circuitry involved in locomo-tor pattern generation: insights from deletions during fictive locomotion,” J. Physiol., 577, Pt. 2, 617–639 (2006).
N. A. Shevtsova and I. A. Rybak, “Organization of flexorextensor interactions in the mammalian spinal cord: insights from computational modelling,” J. Physiol., 594, No. 21, 6117–6131 (2016).
S. N. Markin, M. A. Lemay, B. I. Prilutsky, et al., “Motoneuronal and muscle synergies involved in cat hindlimb control during fictive and real locomotion: a comparison study,” J. Neurophysiol., 107, No. 8, 2057–2071 (2012).
H. Barbeau and S. Rossignol, “The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat,” Brain Res., 514, No. 1, 55–67 (1990).
H. Barbeau and S. Rossignol, “Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs,” Brain Res., 546, No. 2, 250–260 (1991).
A. J. Berger and T. Takahashi, “Serotonin enhances a low voltage-activated calcium current in rat spinal motoneurons,” J. Neurosci., 10, No. 6, 1922–1928 (1990).
C. Chau, H. Barbeau, and S. Rossignol, “Effects of intrathecal alpha1- and alpha2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats,” J. Neurophysiol., 79, No. 6, 2941–2963 (1998).
O. Kiehn, K. T. Sillar, O. Kjaerulff, et al., “Effects of noradrenaline on locomotor rhythm–generating networks in the isolated neonatal rat spinal cord,” J. Neurophysiol., 82, No. 2, 741–746 (1999).
G. B. Miles and K. T. Sillar, “Neuromodulation of vertebrate locomotor control networks,” Physiology (Bethesda), 26, No. 6, 393–411 (2011).
M. Goulding, S. Bourane, L. Garcia-Campmany, et al., “Inhibition down under: an update from the spinal cord,” Curr. Opin. Neurobiol., 26, 161–166 (2014).
J. Zhang, G. M. Lanuza, O. Britz, et al., “V1 and v2b interneurons secure the alternating flexor-extensor motor activity in mice required for limbed locomotion,” Neuron, 82, No. 1, 138–150 (2014).
D. A. McCrea and I. A. Rybak, “Organization of mammalian locomotor rhythm and pattern generation,” Brain Res. Rev., 57, No. 1, 134–146 (2008).
M. Hagglund, K. J. Dougherty, L. Borgius, et al., “Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion,” Proc. Natl. Acad. Sci. USA, 110, No. 28, 11589–11594 (2013).
S. Grillner and A. El Manira, “Current principles of motor control, with special reference to vertebrate locomotion,” Physiol. Rev., 100, No. 1, 271–320 (2020).
E. Marder, D. Bucher, “Central pattern generators and the control of rhythmic movements,” Curr. Biol., 11, No. 23, R986–R996 (2001).
S. Grillner, “Control of locomotion in bipeds, tetrapods, and fish,” Compr. Physiol., Suppl. 2, Handbook of Physiology, The Nervous System, Motor Control: 1179–1236 (2011).
J. Cheng, R. B. Stein, K. Jovanovic, et al., “Identification, localization, and modulation of neural networks for walking in the mudpuppy (Necturus maculatus) spinal cord,” J. Neurosci., 18, No. 11, 4295–4304 (1998).
K. L. Briggman and W. B. Kristan, Jr., “Imaging dedicated and multifunctional neural circuits generating distinct behaviors,” J. Neurosci., 26, No. 42, 10925–10933 (2006).
S. R. Soffe, “Two distinct rhythmic motor patterns are driven by common premotor and motor neurons in a simple vertebrate spinal cord,” J. Neurosci., 13, No. 10, 4456–4469 (1993).
J. C. Liao and J. R. Fetcho, “Shared versus specialized glycinergic spinal interneurons in axial motor circuits of larval zebrafish,” J. Neurosci., 28, No. 48, 12982–12992 (2008).
L. Cangiano and S. Grillner, “Mechanisms of rhythm generation in a spinal locomotor network deprived of crossed connections: the lamprey hemicord,” J. Neurosci., 25, No. 4, 923–935 (2005).
L. Cangiano, R. H. Hill, and S. Grillner, “The hemisegmental locomotor network revisited,” Neuroscience, 210, 33–37 (2012).
A. Berkowitz, “Both shared and specialized spinal circuitry for scratching and swimming in turtles,” J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol.,188, No. 3, 225–234 (2002).
Z.-Z. Hao, L. E. Spardy, E. B. Nguyen, et al., “Strong interactions between spinal cord networks for locomotion and scratching,” J. Neurophysiol., 106, No. 4, 1766–1781 (2011).
A. C. Snyder and J. E. Rubin, “Conditions for multifunctionality in a rhythm generating network inspired by turtle scratching,” J. Math. Neurosci., 5, No. 1, 26 (2015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parker, J., Khwaja, R. & Cymbalyuk, G. Asymmetric Control of Coexisting Slow and Fast Rhythms in a Multifunctional Central Pattern Generator: A Model Study. Neurophysiology 51, 390–399 (2019). https://doi.org/10.1007/s11062-020-09834-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-020-09834-9