Abstract
Endemic goiter is one of the most common endocrine diseases in Russian Federation. There is a strong need for new methods to detect and treat this disease. Analysis of nuclear abnormalities in buccal epithelial cells can be used to estimate overall exposure of patients to various endo- and exogenic factors associated with a disorder. We performed an association study of nuclear abnormalities in buccal epithelial cells and as well polymorphisms of genes encoding superoxide dismutase 1 (SOD1) and superoxide dismutase 2 (SOD2) in patients with endemic goiter. We observed an increased frequency of nuclear abnormalities related to early stages of apoptosis and necrosis in buccal epithelial cells of patients with endemic goiter when compared to cells from healthy individuals. Nuclear abnormalities in patients with endemic goiter are more common in heterozygous carriers of minor alleles of SOD1 G7958A and SOD2 58T>C polymorphisms when compared to homozygous individuals. Furthermore the presence of minor allele of SOD2 60C>T polymorphism in individuals with endemic goiter decreases the frequency of nuclear abnormalities. The results of this study could be used for the further development of effective and low-cost methods of endemic goiter diagnostics.
Similar content being viewed by others

REFERENCES
Abe, K., et al., Association between monoallelic TSHR mutations and congenital hypothyroidism: A statistical approach, Eur. J. Endocrinol., 2018, vol. 178, no. 2, pp. 137–144.
Aceves Avila, F.J., et al., Cyclophosphamide boluses induce micronuclei expression in buccal mucosa cells of patients with systemic lupus erythematosus independent of cytochrome P450 2D6 status, J. Rheumatol., 2004, vol. 31, no. 7, pp. 1335–1339.
Bajaj, J.K., Salwan, P., and Salwan, S., Various possible toxicants involved in thyroid dysfunction: A review, J. Clin. Diagn. Res., 2016, vol. 10, no. 1, pp. FE01–FE03.
Borgstahl, G.E.O., et al., Human mitochondrial manganese superoxide dismutase polymorphic variant Ile58Thr reduces activity by destabilizing the tetrameric interface, Biochemistry, 1996, vol. 35, no. 14, pp. 4287–4297.
Calderon, G.D., et al., Oxidative stress and diabetic retinopathy: development and treatment, Eye, 2017, vol. 31, no. 8, pp. 1122–1130.
Dórea, L.N.T.M., et al., Chromosomal damage and apoptosis in exfoliated buccal cells from individuals with oral cancer, Int. J. Dent., 2012, vol. 2012, p. 457054. https://doi.org/10.1155/2012/457054
Emene, C.C., et al., Analysis of serum cytokines and single-nucleotide polymorphisms of SOD1, SOD2, and CAT in erysipelas patients, J. Immunol. Res., 2017, vol. 2017, p. 2157247. https://doi.org/10.1155/2017/2157247
Evans, J.L., et al., Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes, Endocr. Rev., 2002, vol. 23, no. 5, pp. 599–622.
Faria, A. and Persaud, S.J., Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential, Pharmacol. Ther., 2017, vol. 172, pp. 50–62.
Gredilla, R., et al., Influence of hyper- and hypothyroidism on lipid peroxidation, unsaturation of phospholipids, glutathione system and oxidative damage to nuclear and mitochondrial DNA in mice skeletal muscle, Mol. Cell. Biochem., 2001, vol. 221, nos. 1–2, pp. 41–48.
Hernandez-Saavedra, D. and McCord, J.M., Paradoxical effects of thiol reagents on Jurkat cells and a new thiol-sensitive mutant form of human mitochondrial superoxide dismutase, Cancer Res., 2003, vol. 63, no. 1, pp. 159–163.
Kerr, J.F., Wyllie, A.H., and Currie, A.R., Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics, Br. J. Cancer, 1972, vol. 26, no. 4, pp. 239–257.
Khlifi, R., et al., Cytogenetic abnormality in exfoliated cells of buccal mucosa in head and neck cancer patients in the Tunisian population: Impact of different exposure sources, BioMed Res. Int., 2013, vol. 2013, p. 905252.
Linnane, A.W., et al., Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases, Lancet, 1989, vol. 25, no. 1, pp. 642–645.
Mancini, A., et al., Thyroid hormones, oxidative stress, and inflammation, Mediators Inflammation, 2016, vol. 2016, p. 6757154.
Martin, R.C., et al., Manganese superoxide dismutase gene coding region polymorphisms lack clinical incidence in general population, DNA Cell Biol., 2008, vol. 27, no. 6, pp. 321–323.
Nguyen, T., Nioi, P., and Pickett, C.B., The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress, J. Biol. Chem., 2009, vol. 284, no. 20, pp. 13291–13295.
Nishihara, E., et al., A novel germline mutation of KEAP1 (R483H) associated with a non-toxic multinodular goiter, Front. Endocrinol., 2016, vol. 7, p. 131.
Pouyet, L. and Carrier, A., Mutant mouse models of oxidative stress, Transgenic Res., 2010, vol. 19, no. 2, pp. 155–164.
Rosin, M.P., et al., Localized induction of micronuclei in the oral mucosa of xeroderma pigmentosum patients, Cancer Lett., 1994, vol. 81, no. 1, pp. 39–44.
Sahay, K., et al., Cytomorphometric analysis and morphological assessment of oral exfoliated cells in type 2 diabetes mellitus and healthy individuals: A comparative study, J. Cytol., 2017, vol. 34, no. 1, pp. 27–33.
Siffo, S., et al., Molecular analysis of thyroglobulin mutations found in patients with goiter and hypothyroidism, Mol. Cell. Endocrinol., 2018, vol. 473, pp. 1–16.
Song, X., et al., Association of the glutathione S-transferases M1 and T1 polymorphism with male infertility: A meta-analysis, J. Assisted Reprod. Genet., 2013, vol. 30, no. 1, pp. 131–141.
Targovnik, H.M., Citterio, C.E., and Rivolta, C.M., Iodide handling disorders (NIS, TPO, TG, IYD), Best Pract. Res., Clin. Endocrinol. Metab., 2017, vol. 31, no. 2, pp. 195–212.
Taylor, P.N., et al., Global epidemiology of hyperthyroidism and hypothyroidism, Nat. Rev. Endocrinol., 2018, vol. 14, no. 5, pp. 301–316.
Ueta, Y., et al., Hypothalamic nitric oxide synthase gene expression is regulated by thyroid hormones, Endocrinology, 1995, vol. 136, no. 10, pp. 4182–4187.
Villanueva, I., Alva-Sánchez, C., and Pacheco-Rosado, J., The role of thyroid hormones as inductors of oxidative stress and neurodegeneration, Oxid. Med. Cell. Longevity, 2013, vol. 2013, p. 218145.
Wang, Y., et al., Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling, J. Cell Biol., 2018, vol. 217, no. 6, pp. 1915–1928.
Xu, L.-Y. and Cao, L.-F., GSTT1 genetic polymorphism and susceptibility to childhood acute lymphoblastic leukemia: a meta-analysis, Tumour Biol.:J. Int. Soc. Oncodev. Biol. Med., 2014, vol. 35, no. 2, pp. 1433–1437.
Yi, J.F., et al., Mn-SOD and cuZn-SOD polymorphisms and interactions with risk factors in gastric cancer, World J. Gastroenterol., 2010, vol. 16, no. 37, pp. 4738–4746.
Yildirim, I.H., Yesilada, E., and Yologlu, S., Micronucleus frequency in peripheral blood lymphocytes and exfoliated buccal cells of untreated cancer patients, Genetika, 2006, vol. 42, no. 5, pp. 705–710.
Zhang, H.J., et al., Comparison of effects of two polymorphic variants of manganese superoxide dismutase on human breast MCF-7 cancer cell phenotype, Cancer Res., 1999, vol. 59, no. 24, pp. 6276–6283.
Amirov, E.V., et al., Results of surgical treatment of thyroid nodular structures in women of reproductive age, Med. Almana, pp. 184–186.
Belyaeva, N.N., et al., Evaluation of Cytological and Cytogenetic Status of Human Nose and Oral Mucosa.Methodological Recommendations, Moscow, 2005.
Kochetova, O.V., et al., DIO2, TPO, CYP1A1 and CYP1A2 gene polymorphism in women with thyroid disease, Gig. Sanit., 2014, vol. 3, pp. 52–56.
Kuzmina, V.A., et al., Thyroid diseases in young residents of St. Petersburg, Ross. Semeinyi Vrach, 2011, vol. 3, pp. 24–28.
Machigov, E.A., et al., Glutathione S-transferase genes GSTM1 and GSTT1 in thyroid disease genesis, Proc. 64th Int. Research-to-Practice Conference, Novosibirsk: SibAK, 12 (61) Part I, pp. 19–25.
Petunina, N.A. and Trukhina, L.V., Bolezni shchitovidnoi zhelezy (Thyroid Diseases), Moscow: GEOTAR-Media, 2011.
Sycheva, L.P., Biological significance, detection criteria and variation range of the karyological characteristics in evaluation of human cytogenetic characterization, Med. Genet., 2007, vol. 11, pp. 3–11.
Shilina, M.M., et al., Frequency and distribution of thyroid disease in residents of different geochemical regions of Novosibirsk region, Sib. Med. Zh., 2008, vol. 23, nos. 1–2, pp. 55–58.
Yunusov, A.A., Micronuclei in exfoliated buccal cells of women of reproductive age with thyroid disease, Bull. Kyrg. Russ. Slavic Univ., 2015, vol. 4, pp. 194–196.
Yurchenko, V.V., et al., Micronuclear analysis of human buccal epithelial cells, in Polyorgan Micronuclear Analysis in Environmental and Health Studies, Moscow: Genius, 2007.
Russian Unified Interdepartmental Statistical Information System. 2011. Thyroid Disease Incidence in Russia. https://fedstat.ru/indicator/41717.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study.
Additional information
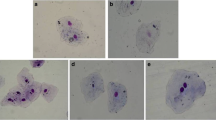
Abbreviations: EG—endemic goiter; ROS—reactive oxygen species; MN—micronuclei; NP—nuclear protrusions; KL—karyolysis; KP—karyopinkosis; KR—karyorrhexis; NV—nuclear vacuolization; NL—nuclear lobes, BN cells—binuclear cells.
About this article
Cite this article
Bisultanova, Z.I., Dzhambetova, L.M., Machigov, E.A. et al. Association Study of Nuclear Abnormalities and Polymorphisms of Oxidative Stress Genes with Endemic Goiter Incidence. Mol. Genet. Microbiol. Virol. 34, 237–243 (2019). https://doi.org/10.3103/S0891416819040025
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0891416819040025