Abstract
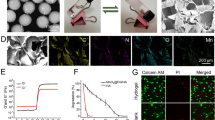
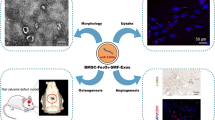
Superparamagnetic iron oxide nanoparticles have been widely used for cell labeling in preclinical and clinical studies, to improve labeling efficiency, particle conjugation and surface modifications are developed, but some modified SPIONs exert side-effect on physiological activity of cells, which cannot be served as ideal cell tracker. In this study, amine-modified silica-coated SPIO (SPIO@SiO2-NH2, SPIO@S-N) nanoparticles were used to label bone marrow derived mesenchymal stem cells (BM-MSCs), then the stem cell potentials were evaluated. It was found BM-MSCs could be efficiently labeled by SPIO@S-N nanoparticles. After labeling, the BM-MSCs viability kept well and the migration ability increased, but the osteogenesis and adipogenesis potentials were not impaired. In steroid associated osteonecrosis (SAON) bone defect model, stem cell implantation was performed by injection of SPIO@S-N labeled BM-MSCs into marrow cavity locally, it was found the SPIO positive cells homed to the periphery of defect region in control group, but were recruited to the defect region in poly lactic-coglycolic acid/tricalcium phosphate (PLGA/TCP) scaffold implantation group. In conclusion, SPIO@S-N nanoparticles promoted migration while retained proliferation and differentiation ability of BM-MSCs, implying this kind of nanoparticles could be served not only an ideal tracking marker but also an accelerator for stem cell homing during tissue repair.




Similar content being viewed by others
References
Andreas K, Georgieva R, Ladwig M, Mueller S, Notter M, Sittinger M, Ringe J (2012) Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials 33:4515–4525
Askari AT et al (2003) Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 362:697–703
Bulte JW et al (2001) Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol 19:1141–1147
Chang YK, Liu YP, Ho JH, Hsu SC, Lee OK (2012) Amine-surface-modified superparamagnetic iron oxide nanoparticles interfere with differentiation of human mesenchymal stem cells. J Orthop Res 30:1499–1506
Chen YC et al (2010) The inhibitory effect of superparamagnetic iron oxide nanoparticle (Ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cells. Toxicol Appl Pharmacol 245:272–279
Coyne DW (2009) Ferumoxytol for treatment of iron deficiency anemia in patients with chronic kidney disease. Expert Opin Pharmacother 10:2563–2568
De Becker A, Riet IV (2016) Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem Cells 8:73–87
Elstner A, Holtkamp N, von Deimling A (2007) Involvement of Hif-1 in desferrioxamine-induced invasion of glioblastoma cells. Clin Exp Metastasis 24:57–66
Fox JM, Chamberlain G, Ashton BA, Middleton J (2007) Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol 137:491–502
Gu L, Li X, Jiang J, Guo G, Wu H, Wu M, Zhu H (2018) Stem cell tracking using effective self-assembled peptide-modified superparamagnetic nanoparticles. Nanoscale
Guzman R et al (2007) Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci USA 104:10211–10216
Hauger O et al (2006) MR evaluation of the glomerular homing of magnetically labeled mesenchymal stem cells in a rat model of nephropathy. Radiology 238:200–210
Howe AK (2011) Cross-talk between calcium and protein kinase A in the regulation of cell migration. Curr Opin Cell Biol 23:554–561
Huang DM et al (2009) The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 30:3645–3651
Hunter AC (2006) Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv Drug Deliv Rev 58:1523–1531. https://doi.org/10.1016/j.addr.2006.09.008
Jasmin GTDS, Louzada RA, Rosado-de-Castro PH, Mendez-Otero R, de Carvalho ACC (2017) Tracking stem cells with superparamagnetic iron oxide nanoparticles: perspectives and considerations. Int J Nanomedicine 12:779–793
Jin R, Lin B, Li D, Ai H (2014) Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: design considerations and clinical applications. Curr Opin Pharmacol 18:18–27
Ju S, Teng G, Zhang Y, Ma M, Chen F, Ni Y (2006) In vitro labeling and MRI of mesenchymal stem cells from human umbilical cord blood. Magn Reson Imaging 24:611–617
Kalber TL et al (2016) Hyperthermia treatment of tumors by mesenchymal stem cell-delivered superparamagnetic iron oxide nanoparticles. Int J Nanomedicine 11:1973–1983
Kim HS, Oh SY, Joo HJ, Son KR, Song IC, Moon WK (2010) The effects of clinically used MRI contrast agents on the biological properties of human mesenchymal stem cells. NMR Biomed 23:514–522
Kim SJ, Lewis B, Steiner MS, Bissa UV, Dose C, Frank JA (2016) Superparamagnetic iron oxide nanoparticles for direct labeling of stem cells and in vivo MRI tracking. Contrast Media Mol Imaging 11:55–64
Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW (2004) Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed 17:513–517
Krahling H, Mally S, Eble JA, Noel J, Schwab A, Stock C (2009) The glycocalyx maintains a cell surface pH nanoenvironment crucial for integrin-mediated migration of human melanoma cells. Pflugers Arch 458:1069–1083
Lei H et al (2015) Stem cell labeling with superparamagnetic iron oxide nanoparticles using focused ultrasound and magnetic resonance imaging tracking. J Nanosci Nanotechnol 15:2605–2612
Leibacher J, Henschler R (2016) Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther 7:7
Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R (2000) Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol 18:410–414
Mahmoudi M, Simchi A, Milani AS, Stroeve P (2009) Cell toxicity of superparamagnetic iron oxide nanoparticles. J Colloid Interface Sci 336:510–518
Matthay MA, Pati S, Lee JW (2017) Concise review: mesenchymal stem (stromal) cells: biology and preclinical evidence for therapeutic potential for organ dysfunction following trauma or sepsis. Stem Cells 35:316–324
Pittenger MF et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Qin L et al (2006) Multiple bioimaging modalities in evaluation of an experimental osteonecrosis induced by a combination of lipopolysaccharide and methylprednisolone. Bone 39:863–871
Qin L et al (2015) Phytomolecule icaritin incorporated PLGA/TCP scaffold for steroid-associated osteonecrosis: proof-of-concept for prevention of hip joint collapse in bipedal emus and mechanistic study in quadrupedal rabbits. Biomaterials 59:125–143
Raheja LF, Genetos DC, Wong A, Yellowley CE (2011) Hypoxic regulation of mesenchymal stem cell migration: the role of RhoA and HIF-1alpha. Cell Biol Int 35:981–989
Schafer R et al (2009) Labeling of human mesenchymal stromal cells with superparamagnetic iron oxide leads to a decrease in migration capacity and colony formation ability. Cytotherapy 11:68–78
Smart N, Riley PR (2008) The stem cell movement. Circ Res 102:1155–1168
Stuwe L et al (2007) pH dependence of melanoma cell migration: protons extruded by NHE1 dominate protons of the bulk solution. J Physiol 585:351–360
Sykova E, Jendelova P (2007) Migration, fate and in vivo imaging of adult stem cells in the CNS. Cell Death Differ 14:1336–1342
Torres ALM, Jelicks L, de Carvalho AC, Spray DC, Mendez-Otero R (2012) Labeling stem cells with superparamagnetic iron oxide nanoparticles: analysis of the labeling efficacy by microscopy and magnetic resonance imaging. Methods Mol Biol 906:239–252
Vora P et al (2012) CXCL1 regulation of oligodendrocyte progenitor cell migration is independent of calcium signaling. Exp Neurol 236:259–267
Wang HH et al (2009) Durable mesenchymal stem cell labelling by using polyhedral superparamagnetic iron oxide nanoparticles. Chemistry 15:12417–12425
Wang YX et al (2010) Low-intensity pulsed ultrasound increases cellular uptake of superparamagnetic iron oxide nanomaterial: results from human osteosarcoma cell line U2OS. J Magn Reson Imaging 31:1508–1513. https://doi.org/10.1002/jmri.22173
Wang C et al (2015) Application of bone marrow mesenchymal stem cells to the treatment of osteonecrosis of the femoral head. Int J Clin Exp Med 8:3127–3135
Wang Q et al (2016) Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 86:11–20
Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H (2009) Calcium flickers steer cell migration. Nature 457:901–905
Xi J, Yan X, Zhou J, Yue W, Pei X (2013) Mesenchymal stem cells in tissue repairing and regeneration: progress and future. Burns Trauma 1:13–20
Xie XH et al (2010) Structural and degradation characteristics of an innovative porous PLGA/TCP scaffold incorporated with bioactive molecular icaritin. Biomed Mater 5:054109
Xie XH, Wang XL, Yang HL, Zhao DW, Qin L (2015) Steroid-associated osteonecrosis: epidemiology, pathophysiology, animal model, prevention, and potential treatments (an overview). J Orthop Translat 3:58–70
Yao D et al (2012) Icaritin, an exogenous phytomolecule, enhances osteogenesis but not angiogenesis—an in vitro efficacy study. PLoS ONE 7:e41264
Yarrow JC, Perlman ZE, Westwood NJ, Mitchison TJ (2004) A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol 4:21
Zhao JW, Gao ZL, Mei H, Li YL, Wang Y (2011) Differentiation of human mesenchymal stem cells: the potential mechanism for estrogen-induced preferential osteoblast versus adipocyte differentiation. Am J Med Sci 341:460–468
Zhao L, Kaye AD, Kaye AJ, Abd-Elsayed A (2018) Stem cell therapy for osteonecrosis of the femoral head: current trends and comprehensive review. Curr Pain Headache Rep 22:41
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81560005 and 81760007), Natural Science Foundation of Guangxi province (2016GXNSFBA380003 and 2018GXNSFAA138052).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yao, D., Liu, Nn. & Mo, Bw. Assessment of proliferation, migration and differentiation potentials of bone marrow mesenchymal stem cells labeling with silica-coated and amine-modified superparamagnetic iron oxide nanoparticles. Cytotechnology 72, 513–525 (2020). https://doi.org/10.1007/s10616-020-00397-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-020-00397-5