Abstract
Background
Silica dioxide nanoparticles (SiONPs) have been used for various medical applications, including therapeutics and imaging, and the use of SiONPs has increased gradually over the years. However, despite an increase in the use of SiONPs, not much is known about mechanism of action of SiONPs and their pulmonary toxicity.
Objective
The present study investigated the pulmonary toxicity of SiONPs and explored the underlying mechanism of action, primarily focusing on thioredoxin-interacting protein (TXNIP)/NOD-like receptor pyrin domain-containing 3 (NLRP3) in SiONPs-treated mice. We investigated the toxic effects of SiONPs in the lung of BALB/c mice administered 5, 10, and 20 mg/kg SiONPs for 3 days.
Results
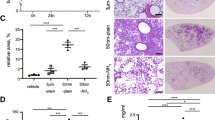
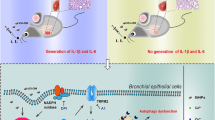
Exposure to SiONPs markedly increased inflammatory cell counts, including those of neutrophils and macrophages, and levels of inflammatory mediators, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α in a dose-dependent manner in the bronchoalveolar lavage fluid. Moreover, the inflammation was verified upon histopathological analysis. In addition, exposure to SiONPs increased the expression of TXNIP in a dose-dependent manner and, in turn, upregulated NLRP3 inflammasome proteins, which subsequently induced IL-1β production.
Conclusion
Collectively, exposure to SiONPs induced inflammation in the lungs of mice, which resulted in the activation of IL-1β production via the TXNIP-NLRP3 axis. Our results provide useful information on the pulmonary toxicity induced by SiONPs and provide insights into the underlying mechanism of action.





Similar content being viewed by others
References
Abais JM et al (2014) Nod-like receptor protein 3 (NLRP3) inflammasome activation and podocyte injury via thioredoxin-interacting protein (TXNIP) during hyperhomocysteinemia. J Biol Chem 289:27159–27168
Bai RX et al (2019) Repression of TXNIP-NLRP3 axis restores intestinal barrier function via inhibition of myeloperoxidase activity and oxidative stress in nonalcoholic steatohepatitis. J Cell Physiol 234:7524–7538
Baron L et al (2015) The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis 6:e1629
Bauer AT et al (2011) Cytotoxicity of silica nanoparticles through exocytosis of von Willebrand factor and necrotic cell death in primary human endothelial cells. Biomaterials 32:8385–8393
Brandenberger C et al (2013) Engineered silica nanoparticles act as adjuvants to enhance allergic airway disease in mice. Part Fibre Toxicol 10:26
Calvert GM, Rice FL, Boiano JM, Sheehy JW, Sanderson WT (2003) Occupational silica exposure and risk of various diseases: an analysis using death certificates from 27 states of the United States. Occup Environ Med 60:122–129
Chen L et al (2018) The toxicity of silica nanoparticles to the immune system. Nanomedicine (Lond) 3:1939–1962
Cohn L, Whittaker L, Niu N, Homer RJ (2002) Cytokine regulation of mucus production in a model of allergic asthma. Novartis Found Symp 248:201–213 (discussion 213–220, 277–282)
Corbalan JJ, Medina C, Jacoby A, Malinski T, Radomski MW (2011) Amorphous silica nanoparticles trigger nitric oxide/peroxynitrite imbalance in human endothelial cells: inflammatory and cytotoxic effects. Int J Nanomed 6:2821–2835
Fahy JV, Dickey BF (2010) Airway mucus function and dysfunction. N Engl J Med 363:2233–2247
Gómez DM, Urcuqui-Inchima S, Hernandez JC (2017) Silica nanoparticles induce NLRP3 inflammasome activation in human primary immune cells. Innate Immun 23:697–708
Gonzalez A, Sahaza JH, Ortiz BL, Restrepo A, Cano LE (2003) Production of pro-inflammatory cytokines during the early stages of experimental Paracoccidioides brasiliensis infection. Med Mycol 41:391–399
Gu C, Liu S, Wang H, Dou H (2019) Role of the thioredoxin interacting protein in diabetic nephropathy and the mechanism of regulating NOD-like receptor protein 3 inflammatory corpuscle. Int J Mol Med 43:2440–2450
Halamoda Kenzaoui B, Chapuis Bernasconi C, Guney-Ayra S, Juillerat-Jeanneret L (2012) Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem J 441:813–821
Hassankhani R et al (2015) In vivo toxicity of orally administrated silicon dioxide nanoparticles in healthy adult mice. Environ Sci Pollut Res Int 22:1127–1132
Kim YS, Chung YH, Seo DS, Choi HS, Lim CH (2018) Twenty-eight-day repeated inhalation toxicity study of aluminum oxide nanoparticles in male Sprague-Dawley rats. Toxicol Res 34:343–354
Ko JW et al (2016) Copper oxide nanoparticle induces inflammatory response and mucus production via MAPK signaling in human bronchial epithelial cells. Environ Toxicol Pharmacol 43:21–26
Kusaka T et al (2014) Effect of silica particle size on macrophage inflammatory responses. PLoS ONE 9:e92634
Lee K et al (2019) Two distinct cellular pathways leading to endothelial cell cytotoxicity by silica nanoparticle size. J Nanobiotechnol 17:24
Leung CC, Yu ITS, Chen W (2012) Silicosis. Lancet 379:2008–2018
Li JJ, Muralikrishnan S, Ng CT, Yung LY, Bay BH (2010) Nanoparticle-induced pulmonary toxicity. Exp Biol Med 235:1025–1033
Liu X, Sun J (2010) Endothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-κB pathways. Biomaterials 31:8198–8209
Mohamed IN et al (2014) Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia 57:413–423
Mousseau F, Berret JF (2018) The role of surface charge in the interaction of nanoparticles with model pulmonary surfactants. Soft Matter 14:5764–5774
Murugadoss S et al (2017) Toxicology of silica nanoparticles: an update. Arch Toxicol 91:2967–3010
Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH (2010) The nanosilica hazard: another variable entity. Part Fibre Toxicol 7:39
Park JW et al (2016) Copper oxide nanoparticles aggravate airway inflammation and mucus production in asthmatic mice via MAPK signaling. Nanotoxicology 10:445–452
Sansbury BE, Spite M (2016) Resolution of acute inflammation and the role of resolvins in immunity, thrombosis, and vascular biology. Circ Res 119:113–130
Sayan M, Mossman BT (2016) The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part Fibre Toxicol 13:51
Sharma B, McLeland CB, Potter TM, Stern ST, Adiseshaiah PP (2018) Assessing NLRP3 inflammasome activation by nanoparticles. Methods Mol Biol 1682:135–147
Shen ML et al (2016) Luteolin attenuates airway mucus overproduction via inhibition of the GABAergic system. Sci Rep 6:32756
Shen Y et al (2018) Management of airway mucus hypersecretion in chronic airway inflammatory disease: Chinese expert consensus (English edition). Int J Chron Obstruct Pulmon Dis 13:399–407
Shin IS et al (2015) Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling. J Pineal Res 58:50–60
Silva-Carvalho R et al (2019) Inhalation of bacterial cellulose nanofibrils triggers an inflammatory response and changes lung tissue morphology of mice. Toxicol Res 35:45–63
Xiao YD et al (2016) Thioredoxin-interacting protein mediates NLRP3 inflammasome activation involved in the susceptibility to ischemic acute kidney injury in diabetes. Oxid Med Cell Longev 2016:2386068
Yazdi AS et al (2010) Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1α and IL-1β. Proc Natl Acad Sci USA 107:19449–19454
Zhang J, Zhang H, Deng X, Zhang Y, Xu K (2017) Baicalin protects AML-12 cells from lipotoxicity via the suppression of ER stress and TXNIP/NLRP3 inflammasome activation. Chem Biol Interact 278:189–196
Zhigang Xu et al (2017) Multifunctional silica nanoparticles as a promising theranostic platform for biomedical applications. Mater Chem Front 1:1257–1272
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [NRF-2016R1D1A2B04936124].
Author information
Authors and Affiliations
Contributions
J-OL, I-SS, and J-CK designed the experimental approach and wrote the manuscript. J-WK, H-CK, and J-DH refined the experimental protocols. T-YJ, W-IK, and S-WP performed the animal experiments, biochemical analysis, and histopathological examination. Statistical analyses were done by W-KY. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Je-Oh Lim, Je-Won Ko, Tae-Yang Jung, Woong-Il Kim, So-Won Pak, In-Sik Shin, Won-Kee Yun, Hyoung-Chin Kim, Jeong-Doo Heo, and Jong-Choon Kim declare that they have no conflict of interest.
Human and animal rights
The experimental animal facility and study protocols were approved by the Institutional Animal Care and Use Committee of Chonnam National University. The animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals (NIH, 1978) and the National Animal Welfare Law of the Republic of Korea.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lim, JO., Ko, JW., Jung, TY. et al. Pulmonary inflammation caused by silica dioxide nanoparticles in mice via TXNIP/NLRP3 signaling pathway. Mol. Cell. Toxicol. 16, 245–252 (2020). https://doi.org/10.1007/s13273-020-00080-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-020-00080-y